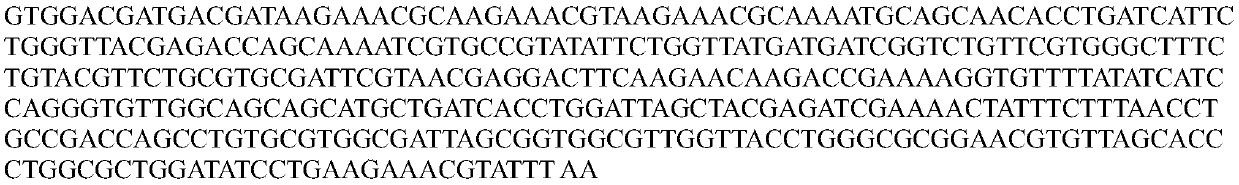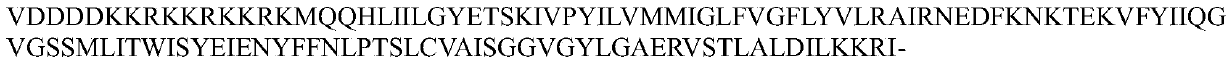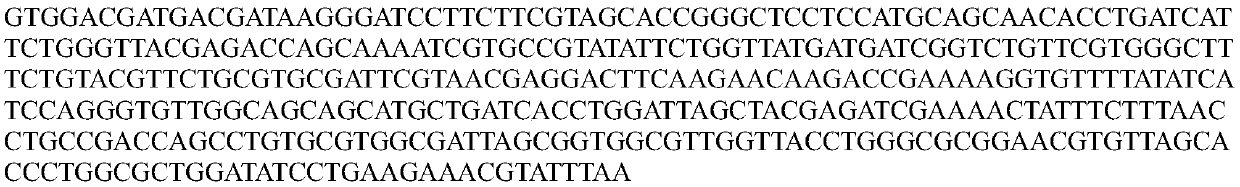Helicobacter pylori bacteriophage endolysins and preparation method thereof
A Helicobacter pylori, phage lysing enzyme technology, applied in the biological field, can solve the problem of weak, cracking Helicobacter pylori, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Preparation of Helicobacter pylori phage lyase containing polycation nonapeptide
[0058] 1. Construction of Helicobacter pylori phage lyase expression vector and engineering bacteria containing polycationic nonapeptide
[0059] 1. Construction of the nucleotide sequence of Helicobacter pylori phage lyase comprising polycation nonapeptide: the polypeptide and nucleotide sequences of polycation nonapeptide and Helicobacter pylori phage lyase are as follows respectively (Seq ID No.7 and Seq ID No. 8, Seq ID No. 11 and Seq ID No. 13). According to the codon preference of Escherichia coli, the nucleotides comprising the natural Helicobacter pylori phage lyase are optimized. This optimization method is a technique known to those skilled in the art. For example, see the Journal of Inner Mongolia University: Escherichia coli and yeast codon usage Compare, 2006, vOI.37, NO.1:34-39; the final nucleotide sequence is as follows (Seq ID No.12). Add polycation nonapeptide sequence...
Embodiment 2
[0083] Preparation of Helicobacter pylori Phage Lyase Containing Small Hydrophobic Peptides
[0084] 1. Construction of Helicobacter pylori phage lyase expression vector and engineering bacteria containing hydrophobic small peptides
[0085] 1. Construct the nucleotide sequence of the Helicobacter pylori phage lyase containing the hydrophobic small peptide: the polypeptide and nucleotide sequences of the hydrophobic small peptide and the Helicobacter pylori phage lyase are as follows (Seq ID No.9 and Seq ID No. 10, Seq ID No. 11 and Seq ID No. 13). According to the codon preference of Escherichia coli, the nucleotide containing the natural Helicobacter pylori phage lyase was optimized, and the final nucleotide sequence was obtained as follows (Seq ID No.12). Add hydrophobic small peptide sequence (gene sequence is Seq ID No.9) and enterokinase cleavage site VDDDDK sequence (gene sequence is Seq ID No.5) to the 5' end of Helicobacter pylori phage lyase to obtain hydrophobic sm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com