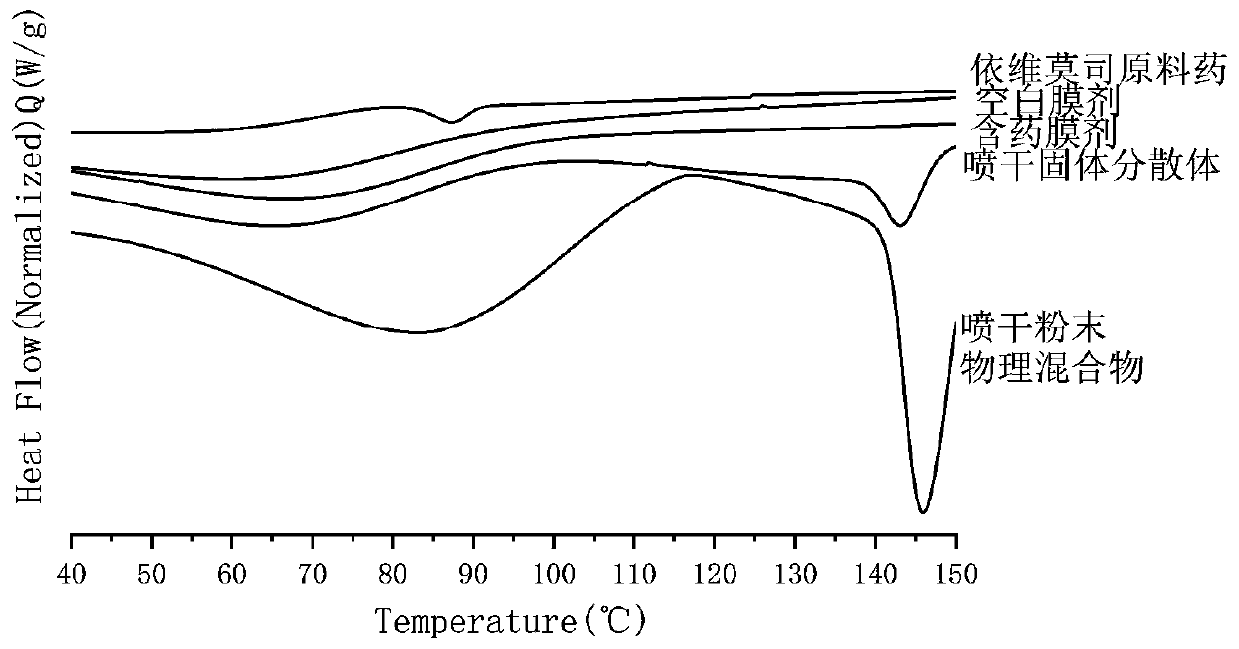
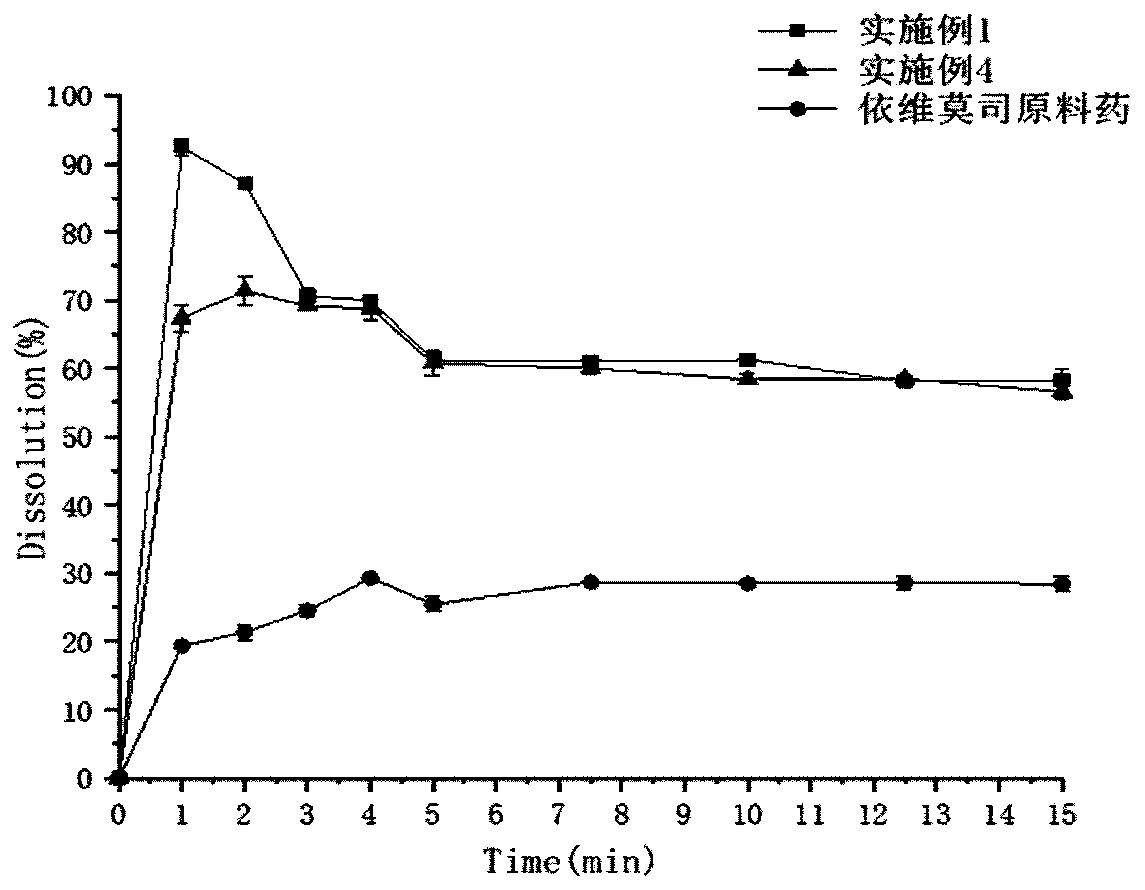
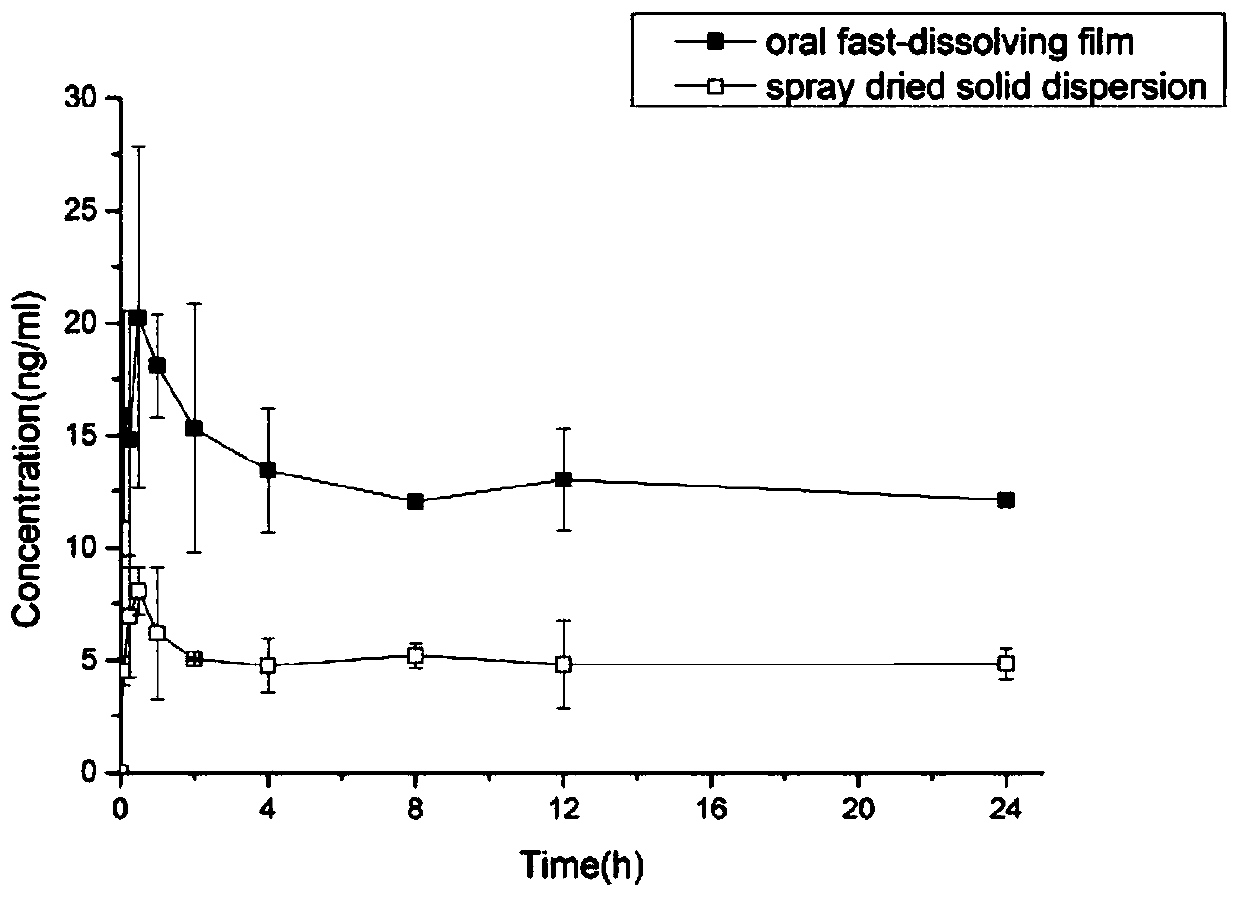
Everolimus oral membrane agent and preparation method thereof
An oral film, everolimus technology, applied in the field of medicine, can solve the problems of large individual differences and low bioavailability of everolimus, achieve the effects of light weight, avoid first-pass effect, and improve preparation stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Prescription: Everolimus: 20mg
[0068] HPMCE5: 240mg
[0069] PEG400: 144mg
[0070] Gum Arabic: 96mg
[0071] Preparation:
[0072] Disperse HPMCE5 in 4ml of 75% ethanol and stir in a 60°C water bath until dissolved;
[0073] While stirring, add a small amount of gum arabic to the HPMCE5 aqueous solution several times, and stir in a 60°C water bath until it dissolves;
[0074] Add the solution obtained in step (2) into PEG400, stir and mix well.
[0075] After the solution obtained in step (3) was cooled to room temperature, everolimus was added, and stirred for 10 minutes to mix well.
[0076] The solution obtained in step (4) is left to defoam. Cast the formed non-bubble viscous liquid on a mold, dry it in vacuum at 50°C for 12 hours, take out the film, cut it into 2×2cm size, and get it.
Embodiment 2
[0078] Prescription: Everolimus: 20mg
[0079] HPMCE5: 240mg
[0080] Glycerin: 96mg
[0081] Gum Arabic: 96mg
[0082] Preparation:
[0083] Disperse HPMCE5 in 4ml of 75% ethanol and stir in a 60°C water bath until dissolved;
[0084] While stirring, add a small amount of gum arabic to the HPMCE5 aqueous solution several times, and stir in a 60°C water bath until it dissolves;
[0085] Add the solution obtained in step (2) into glycerin, stir and mix well.
[0086] After the solution obtained in step (3) was cooled to room temperature, everolimus was added, and stirred for 10 minutes to mix well.
[0087] The solution obtained in step (4) is left to defoam. Cast the formed viscous suspension without bubbles on a mold, dry it in vacuum at 50°C for 12 hours, take out the film, cut it into 2×2cm size, and get it.
Embodiment 3
[0089] Prescription: Everolimus: 20mg
[0090] Methylcellulose (MC): 144mg
[0091] Glycerin: 48mg
[0092] Gum Arabic: 96mg
[0093] Preparation:
[0094] Weigh the prescribed amount of methylcellulose, glycerin, and gum arabic, and disperse them in 1ml of purified water, heat in a water bath at 60°C, stir until dissolved, and cool to room temperature when the solution is clear.
[0095] Weigh the prescribed amount of everolimus and dissolve it in 3ml of absolute ethanol, stir until dissolved.
[0096] Add the liquid medicine obtained in step (2) into the solution obtained in step (1) under constant stirring, and stir for 30 minutes to mix well.
[0097] The solution obtained in step (3) is left to defoam. Cast the formed viscous liquid without bubbles on the mold, dry it in vacuum at 50°C for 12 hours, take out the film, cut it into the required size, and get it.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com