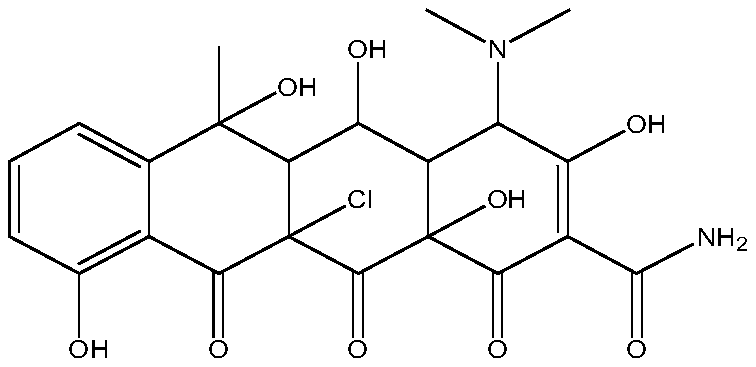
Preparation method of chlorinated oxytetracycline
A technology of oxytetracycline chloride and formula, applied in the field of preparation of oxytetracycline chloride, can solve the problems of high risk, high processing cost, explosion, etc., and achieve simple operation and post-processing, economical and environmentally friendly process, and economical process Another effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A preparation method of oxytetracycline, comprising the following steps:
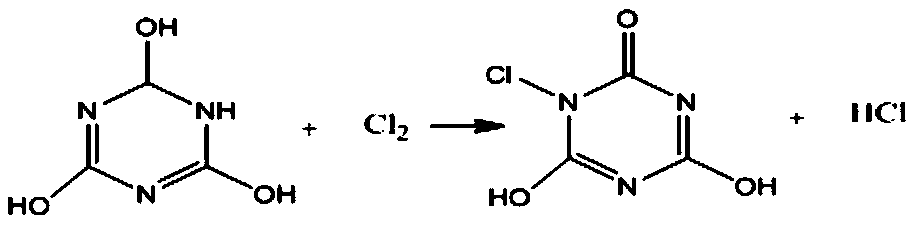
[0033] (1) Add 350g of purified water into a 500ml four-necked bottle equipped with a thermometer and mechanical stirring, heat up to 55-60°C, add 12.9g of pulverized cyanuric acid at one time and stir to completely dissolve;
[0034] (2) Slowly feed chlorine into the cyanuric acid solution obtained in step (1) four-necked bottle, the chlorine quality is controlled at 6.8~7.1g, and the solution pH value is controlled between 2.0~2.5 for 1.5h;
[0035] (3) After the reaction is completed, heat-filter the solid-liquid mixture obtained in step (2), rinse the filter cake with 20 g of 55-60°C hot water for 1-2 times, and dry it in an oven at 70-80°C to obtain monochlorine 13.54g of isocyanuric acid solid is used for later use. After testing, the solid liquid phase content of monochloroisocyanuric acid is 96.45%, and the molar yield is 79.85%;
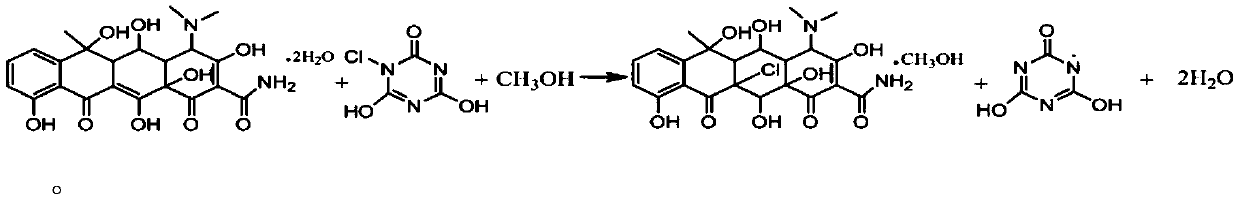
[0036] (4) Add 300g methanol to another 500ml four-neck...
Embodiment 2
[0040] A preparation method of oxytetracycline, comprising the following steps:
[0041] (1) Add 350g of purified water into a 500ml four-necked bottle equipped with a thermometer and mechanical stirring, heat up to 55-60°C, add 12.9g of pulverized cyanuric acid at one time and stir to completely dissolve;
[0042] (2) Slowly feed chlorine into the cyanuric acid solution obtained in step (1) four-necked bottle, the quality of chlorine is controlled at 6.8~7.1g, and the pH value of the solution is controlled between 2.5~3.0 for 1.5h;
[0043] (3) After the reaction is completed, heat-filter the solid-liquid mixture obtained in step (2), rinse the filter cake with 20 g of 55-60°C hot water for 1-2 times, and dry it in an oven at 70-80°C to obtain monochlorine 15.83g of isocyanuric acid solid is used for later use. After testing, the solid-liquid phase content of monochloroisocyanuric acid is 98.30%, and the molar yield is 95.51%;
[0044] (4) Add 300g methanol to another 500ml...
Embodiment 3
[0048] A preparation method of oxytetracycline, comprising the following steps:
[0049] (1) Add 350g of purified water into a 500ml four-necked bottle equipped with a thermometer and mechanical stirring, heat up to 55-60°C, add 12.9g of pulverized cyanuric acid at one time and stir to completely dissolve;
[0050] (2) Slowly feed chlorine into the cyanuric acid solution obtained in step (1) four-necked bottle, the chlorine quality is controlled at 6.8~7.1g, and the solution pH value is controlled between 3.0~3.5 for 1.5h;
[0051] (3) After the reaction is completed, heat-filter the solid-liquid mixture obtained in step (2), rinse the filter cake with 20 g of 55-60°C hot water for 1-2 times, and dry it in an oven at 70-80°C to obtain monochlorine 14.39g of isocyanuric acid solid is used for later use. After testing, the solid liquid phase content of monochloroisocyanuric acid is 97.28%, and the molar yield is 85.60%;
[0052](4) Add 300g methanol to another 500ml four-necke...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com