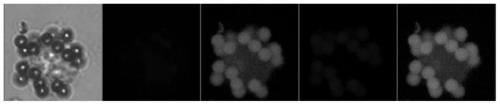
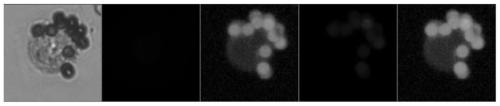
Liquid phase staining identification method used for cells and needing extremely small cell quantity
A dyeing method and identification method technology, which is applied to the determination/inspection of microorganisms, biochemical equipment and methods, measuring devices, etc., can solve the problems of lack of cell integrity, easy loss of gene components, loss of accuracy of results, etc. Removal of cell rupture and loss, promising market application, reducing the effect of magnetic bead processing steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1. Raw materials and instruments: MCF7 breast cancer cell line cells, magnetic capture apparatus (Yichang Micron Silicon Valley Life Science and Technology Co., Ltd.), sample diluent (Yichang Micron Silicon Valley Life Science and Technology Co., Ltd.), cell staining solution.
[0032] The formula of cell staining solution is shown in Table 1
[0033] Table 1
[0034] components volume Cell Binding Solution 3 20ul Cell Binding Solution 4 2ul Cell staining solution 1 2.5ul Cell staining solution 2 2.5ul cell preservation solution Make up to 100ul
[0035] Note: In Table 1, cell staining solution 1 is the secondary antibody that recognizes the primary antibody of blood cells and is labeled with fluorescent Alexa Fluor568; cell staining solution 2 is the secondary antibody that recognizes the primary antibody of keratin and is labeled with fluorescent Alexa Fluor488; cell binding Solution 3 is various primary antibodies to ke...
Embodiment 2
[0046] 1. Raw materials and instruments: circulating tumor cells in the blood of lung cancer patients, magnetic trapping apparatus (Yichang Micron Silicon Valley Life Science and Technology Co., Ltd.), sample diluent (Yichang Micron Silicon Valley Life Science and Technology Co., Ltd.), cell staining solution (recipe as shown in Example Table 1 in 1).
[0047] 2. Experimental steps:
[0048] (1) Release the circulating tumor cells of lung cancer captured by the magnetic trap into a new culture dish filled with clean cell buffer (phosphate buffer);
[0049] (2) After sucking off the liquid part, take 90ul of the sample dilution and add it dropwise to the center of the release tank without drying, and let the cells stand at room temperature for 10 minutes;
[0050] (3) Add 80 ul of Cell Binding Solution 2 (cell buffer containing 1% BSA) dropwise to the center of the release tank, and let the cells stand at room temperature for 3 minutes; slowly discard the solution along the we...
Embodiment 3
[0056] 1. Raw materials and instruments: circulating tumor cells of breast cancer patients, magnetic trapping apparatus (Yichang Micron Silicon Valley Life Science and Technology Co., Ltd.), sample diluent (Yichang Micron Silicon Valley Life Science and Technology Co., Ltd.), cell staining solution (recipe as in Example Table 1 in 1).
[0057] 2. Experimental steps:
[0058] (1) The circulating tumor cells of breast cancer patients captured by the magnetic trap are released and kept in a new petri dish tank filled with clean cell buffer (phosphate buffer);
[0059] (2) After sucking off the liquid part, take 90ul of the sample dilution and add it dropwise to the center of the release tank without drying, and let the cells stand at room temperature for 10 minutes;
[0060] (3) Add 80 ul of Cell Binding Solution 2 (cell buffer containing 1% BSA) dropwise to the center of the release tank, and let the cells stand at room temperature for 3 minutes; slowly discard the solution alo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com