Unsymmetrical naphthalene-pyrrole hybrid diarylethene compound and its application
A diarylethene, asymmetric technology, applied in the direction of organic chemistry, organic chemical methods, chemical instruments and methods, etc., to achieve the effect of low cost, mature reaction conditions, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Compound 1 {1-(2-methyl-1-naphthyl), 2[-1,5-dimethyl-2-cyano-pyrrol-4-yl]} preparation process of perfluorocyclopentene
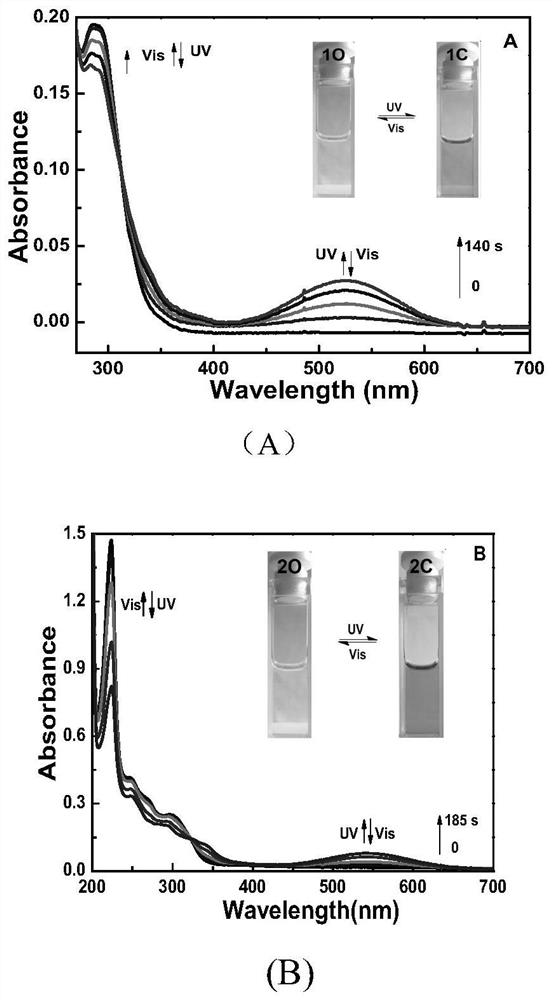
[0028] In the molecular structure formula, when R is cyano (-CN), the photochromic compound 1O is formed, and its name is: {1-(2-methyl-1-naphthyl), 2[-1, The structural formula of 5-dimethyl-2-cyano-pyrrol-4-yl]} perfluorocyclopentene is as follows:
[0029]
[0030] The synthetic scheme of this novel perfluorocyclopentene diarylethene compound is shown in Scheme1
[0031] The synthetic scheme of compound 10 is shown in formula Scheme 1:
[0032]
[0033] Concrete synthetic steps are as follows:
[0034] 1. 4-bromo-1,5-dimethyl-2-cyanopyrrole (2)
[0035] Under ice-bath conditions, dissolve 1,5-dimethyl-2-cyanopyrrole (5.0-6.0 g, 40-50 mmol) in a single-necked flask filled with 100.0 mL of acetic acid, and use a constant pressure dropping funnel to Slowly add 20.0 mL of acetic acid containing 2.2 mL of liquid bromine dropwise, stir and rea...
Embodiment 2
[0044] Synthesis of Compound 2{1-(2-methyl-1-naphthyl,2-[1,5-dimethyl-2-formamido-pyrrol-4-yl]}perfluorocyclopentene (2O) process:
[0045] In the molecular structure formula, when R=-CONH 2 , which constitutes the compound {1-(2-methyl-1-naphthyl, 2-[1,5-dimethyl-2-formamido-pyrrol-4-yl]} perfluorocyclopentene, structural formula as follows:
[0046]
[0047] Compound {1-(2-methyl-1-naphthyl, 2-[1,5-dimethyl-2-formamido-pyrrol-4-yl]} The synthetic scheme of perfluorocyclopentene is shown in the formula Scheme 2 shows:
[0048]
[0049] Concrete synthetic steps are as follows:
[0050] At room temperature, dissolve compound 1O (0.44~0.65g, 1.0~1.5mmol) in DMSO solution, add 0.4molL -1 K 2 CO 3 solution and 2.0mL H 2 o 2 , after reacting for 12 hours, dilute with water and filter with suction to obtain white solid compound 2O (0.25-0.42 g, 0.55-0.92 mmol). Yield: 55.6-65.2%.
[0051] Product structure identification: 1 H-NMR (400MHz, CDCl 3 ):δ1.86(s,3H),2.29(...
Embodiment 3
[0053] Staining of HeLa cells:
[0054] At 37°C, 5% carbon dioxide and 95% air. HeLa cells were placed in a cell culture dish in 20 mL of 10% DMEM bovine serum and allowed to persist overnight before experiments. After the HeLa cells were washed with phosphate-buffered saline (PBS), Compound 1 and Compound 2 (20 μM) were incubated in the culture medium for 30 minutes, respectively. After washing the HeLa cells with PBS three times, the cells were imaged with an Olympus FV1000 laser confocal laser scanning microscope, excited with a 405nm laser, and the fluorescence signals at 400-500nm in the blue channel were collected.
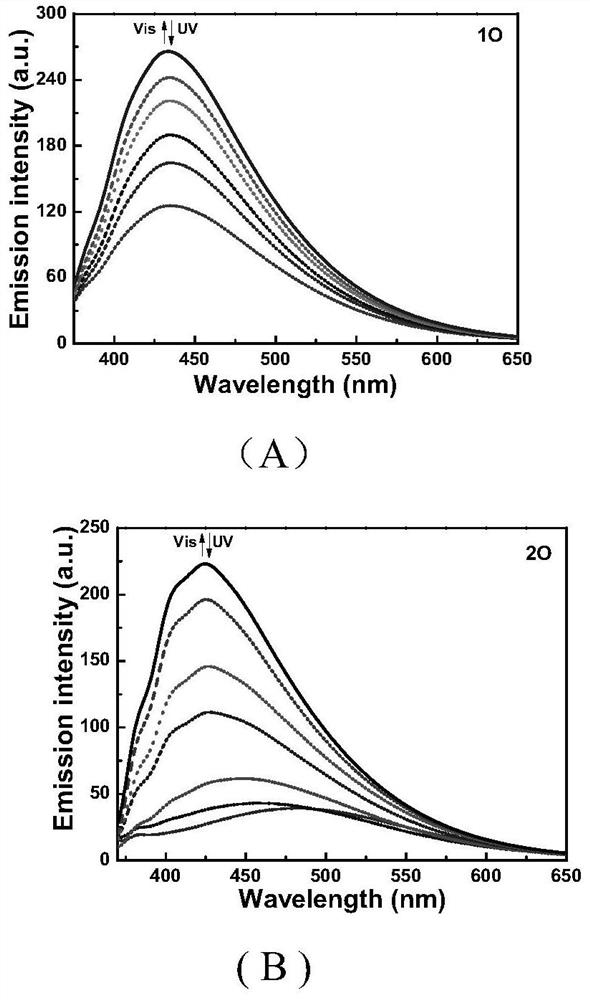
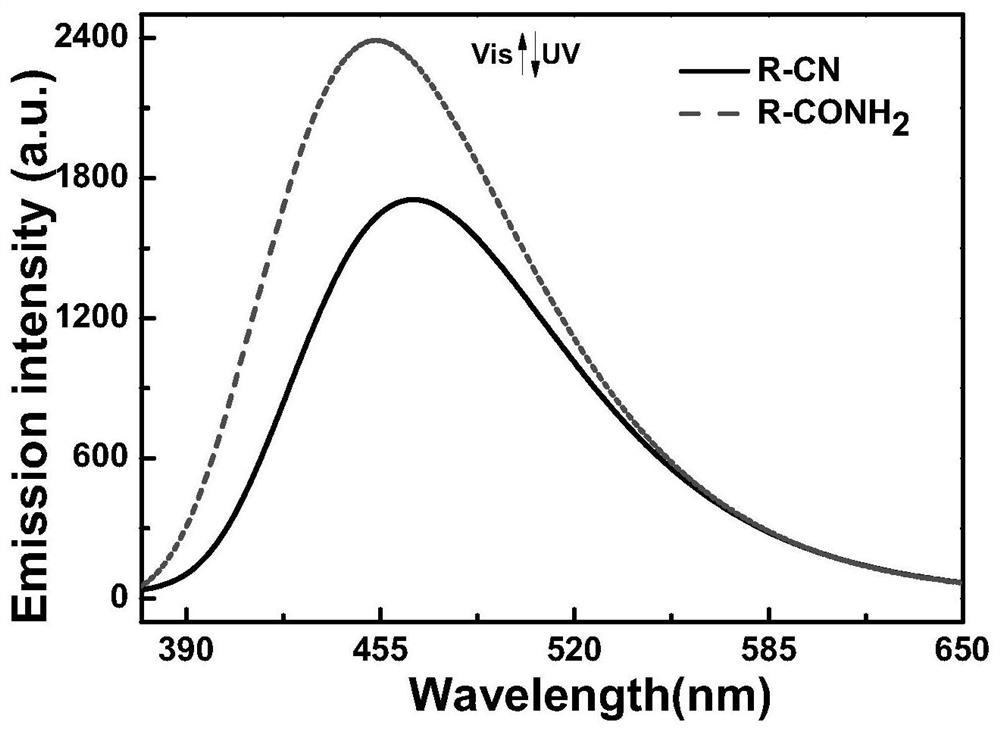
[0055] The main uses of the naphthalene ring-pyrrole mixed-type photochromic perfluorocyclopentene compound molecule of the present invention are: using its good photochromic, thermal stability and other properties, it can be used as an optical storage medium or an optical control switch Component; Utilizing the fluorescent properties of compound molecules...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com