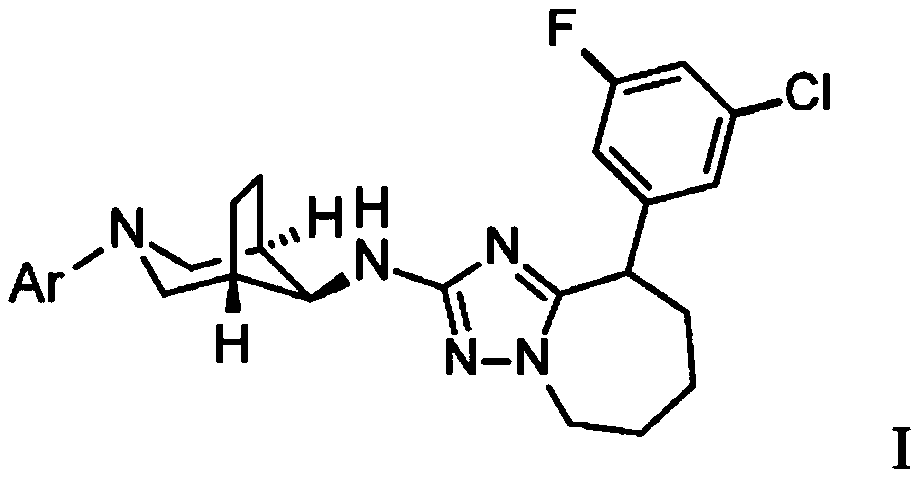
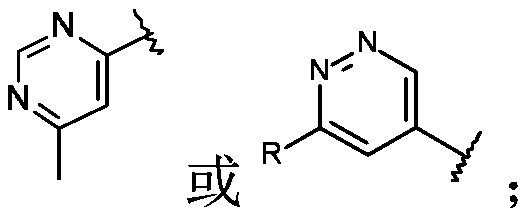
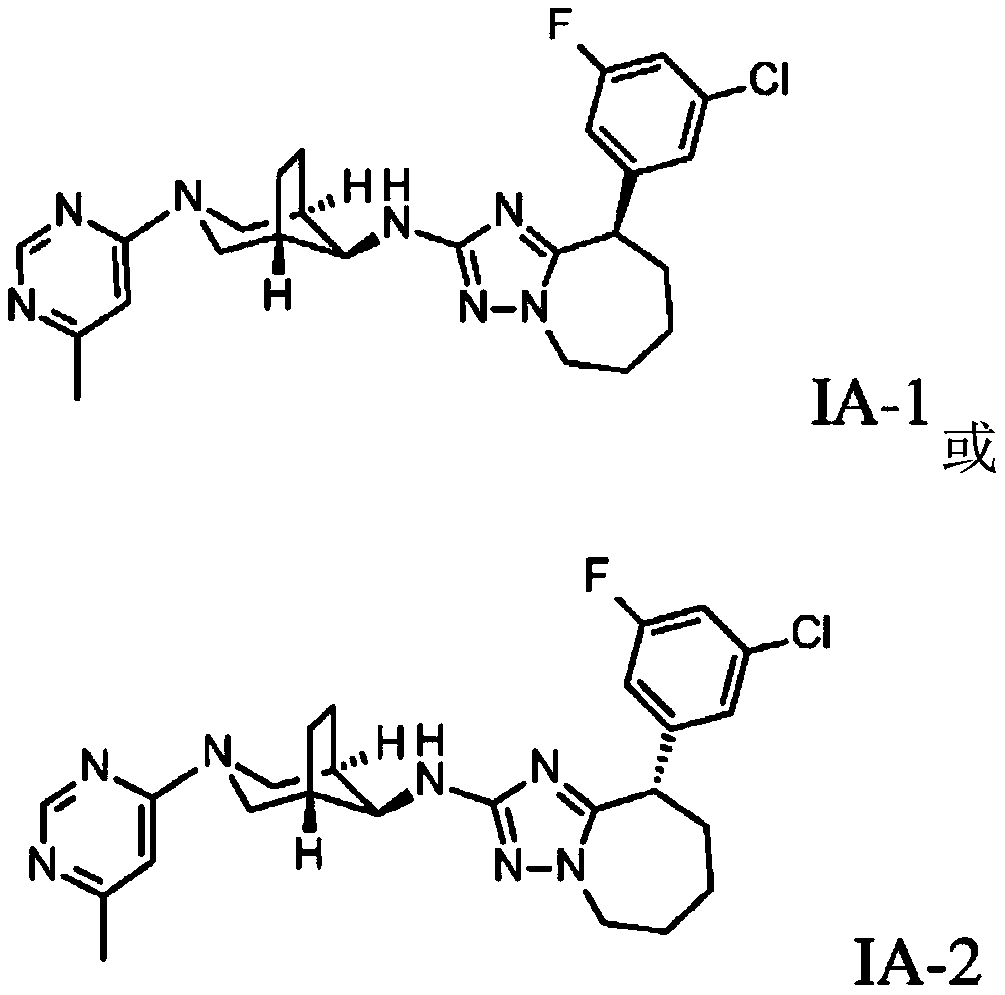
Triazolo-azepine derivatives
A triazolo and azabicyclic technology, applied in the field of triazolo-aza* derivatives, can solve the problem of selectivity reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0134] 1.1) generally
[0135] · Analytical method:
[0136] a) HPLC (method LCMS_fast gradient)
[0137] - Column: Agilent Zorbax Eclipse Plus C18, Fast Resolution HT, 2.1x30 mm, 1.8 μm, part number 959731-902
[0138] - Solvent A: Water 0.01% Formic Acid; Solvent B: Acetonitrile (MeCN)
[0139] -gradient:
[0140] time [min] Flow rate [ml / min] %A %B initial 0.8 97 3 0.2 1.0 97 3 1.7 1.0 3 97 2.0 1.0 3 97 2.1 1.0 97 3
[0141] · abbreviation:
[0142] The following abbreviations are used in the experimental section:
[0143] tBuONa=sodium tert-butoxide;
[0144] DMF = dimethylformamide;
[0145] EtOAc = ethyl acetate;
[0146] EtOH = ethanol;
[0147] Et 3 N=triethylamine;
[0148] LDA = lithium diisopropylamide;
[0149] MeOH=methanol;
[0150] Me 2 SO = dimethyl sulfoxide;
[0151] RT = room temperature, 20-25°C;
[0152] TFA = trifluoroacetic acid;
[0153] THF = tetrahydrofuran;
[0154] TBME = ...
Embodiment 1 and 2
[0203] (9R)-9-(3-chloro-5-fluoro-phenyl)-N-[(1S,5R,8S)-3-(6-methylpyrimidin-4-yl)-3-azabicyclo [3.2.1] Oct-8-yl]-6,7,8,9-tetrahydro-5H-[1,2,4]triazolo[1,5-a]azepine -2-amine
[0204] with
[0205] (9S)-9-(3-chloro-5-fluoro-phenyl)-N-[(1S,5R,8S)-3-(6-methylpyrimidin-4-yl)-3-azabicyclo [3.2.1] Oct-8-yl]-6,7,8,9-tetrahydro-5H-[1,2,4]triazolo[1,5-a]azepine -2-amine
[0206]
[0207] Using intermediate (3.1)(1R,5S,8S)-3-(6-methylpyrimidin-4-yl)-3-azabicyclo[3.2.1]oct-8-amine with compound 2-bromo- 9-(3-Chloro-5-fluoro-phenyl)-6,7,8,9-tetrahydro-5H-[1,2,4]triazolo[1,5-a]azepine The general procedure of the Buchwald coupling between (2), followed by chiral HPLC separation of the enantiomers, prepared 13 mg of (9R)-9-(3-chloro-5-fluoro-phenyl)-N- [(1S,5R,8S)-3-(6-methylpyrimidin-4-yl)-3-azabicyclo[3.2.1]oct-8-yl]-6,7,8,9-tetra Hydrogen-5H-[1,2,4]triazolo[1,5-a]azepine -2-Amine as a white solid (MS(ES+) m / z: 482.2[(M+H) + ]), and 13 mg of (9S)-9-(3-chloro-5-fluoro-pheny...
Embodiment 3 and 4
[0209] (9R)-9-(3-chloro-5-fluoro-phenyl)-N-[(1S,5R,8S)-3-(6-methoxypyridazin-4-yl)-3-aza Bicyclo[3.2.1]oct-8-yl]-6,7,8,9-tetrahydro-5H-[1,2,4]triazolo[1,5-a]azepine -2-amine
[0210] with
[0211] (9S)-9-(3-chloro-5-fluoro-phenyl)-N-[(1S,5R,8S)-3-(6-methoxypyridazin-4-yl)-3-aza Bicyclo[3.2.1]oct-8-yl]-6,7,8,9-tetrahydro-5H-[1,2,4]triazolo[1,5-a]azepine -2-amine
[0212]
[0213] Using intermediate (3.2)(1R,5S,8S)-3-(6-methoxypyridazin-4-yl)-3-azabicyclo[3.2.1]oct-8-amine with compound 2- Bromo-9-(3-chloro-5-fluoro-phenyl)-6,7,8,9-tetrahydro-5H-[1,2,4]triazolo[1,5-a]azepine The general procedure of the Buchwald coupling between (2), followed by chiral HPLC separation of the enantiomers, prepared 33 mg of (9R)-9-(3-chloro-5-fluoro-phenyl)-N- [(1S,5R,8S)-3-(6-Methoxypyridazin-4-yl)-3-azabicyclo[3.2.1]oct-8-yl]-6,7,8,9 -Tetrahydro-5H-[1,2,4]triazolo[1,5-a]azepine -2-Amine as a white solid (MS(ES+) m / z: 498.3[(M+H) + ]), and 32 mg of (9S)-9-(3-chloro-5-fluoro-phenyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com