Cell membrane penetrating conjugates
A technology of conjugates and cells, applied in the direction of hybrid peptides, peptides, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
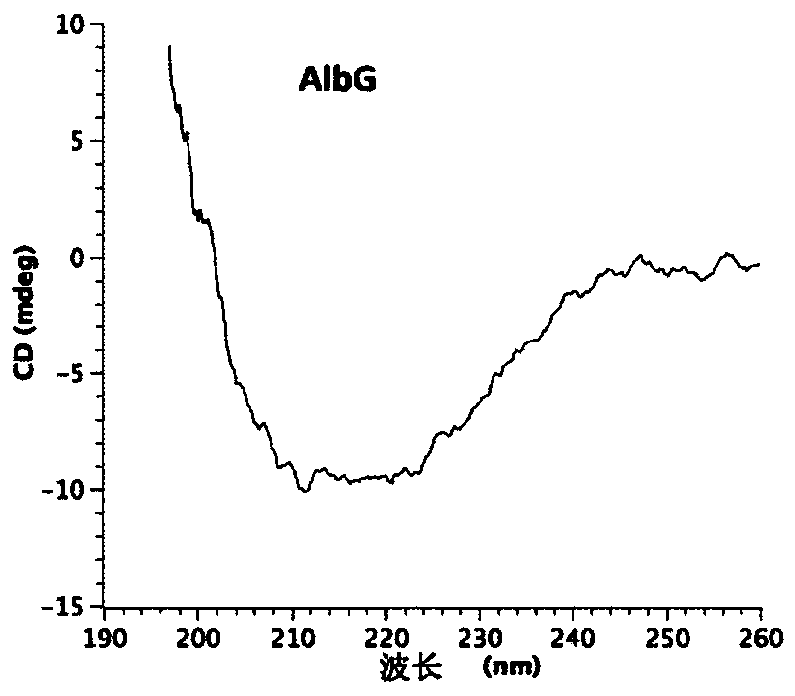
[0320] Plasmid / Protein Research -The plasmid containing the AlbG gene shown in SEQ ID NO:19 was obtained by a previously published method (Vetting et al.Acta Crystallogr.Sect.FStruct.Biol.Cryst.Commun.2011:67(3):296- 302, the contents of which are incorporated herein by reference, and specific details are provided below).
[0321] The open reading frame of AlbG was amplified by standard PCR techniques using X albilineans (ATCC 29184; Pieretti et al., BMC Genomics, 2009, 10:616, 1-15) chromosomal DNA as a template. Oligonucleotides AlbGF (5'-ATCCCGCTCATATGCCGGCCAAGACCCTTG-3') and AlbGR (5'-ATCCCGCTCTCGAGTCAATCGGACAGCTCGATATC-3') containing NdeI and XhoI restriction sites, respectively, were used. The PCR fragment was cloned into pET-28a(+) and recombinant AlbG with a thrombin-cleavable N-terminal His6 tag was expressed in E. coli strain BL21(DE3). For shake flask growth, 10 ml of overnight culture was inoculated into 1 liter of Luria Broth supplemented with kanamycin (35 μg / ...
Embodiment 2
[0327] Toxicity assay - An industry standard MTT assay (from Sigma Aldrich) was performed to analyze the toxicity of β-helical protein-cytotoxic drug conjugates to mammalian cells such as HeLa cells. Cells were cultured by using standard protocols. One million cells were seeded in a confocal plate (1 cm dish) and grown for 6-8 h. A mixture of cytotoxic drug and EfsQNR protein (mixed in a 2:1 ratio) was added to the cells and incubated at room temperature for 15 min to 24 h to 72 h. After incubation, cells were washed with PBS and treated with MTT, and further incubated for 24 hours. Cells were then washed and analyzed for absorbance at 570 nm. Viable cells with active metabolism convert MTT to the purple formazan product with an absorbance maximum near 570nm. Dead cells cannot convert MTT to formazan, therefore, by analyzing the absorbance value at 570nm, the percentage of live cells for a given protein or any other molecule can be calculated.
Embodiment 3
[0329] Labeling proteins with fluorescent dyes- Fluorescent dyes are considered as an example of functional molecules to study the cell membrane penetration ability of conjugates. Recombinant proteins are labeled with dyes by performing a series of reactions under dark conditions. The protein to be labeled is collected in PBS buffer (1X and pH 7.3), and the concentration of the dye collected is 2-3 times that of the protein. Proteins were added to 0.1 M sodium carbonate buffer (pH 8.5) followed by dye addition. The dye was added very slowly (3 μl each) to the buffer containing the protein, which was kept on ice with occasional shaking. The resulting solution was wrapped in aluminum foil to keep out any light. The solution was stirred at room temperature for 1 hour and then purified by gel filtration by passing it through a desalting column or a PD 10 column with 1X PBS buffer. Characterize the resulting labeled protein using UV-Vis spectrophotometry to determine the dye t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com