Etomidate injection preparation and preparation method thereof
A technology for etomidate and injection preparations, applied in the field of pharmaceutical preparations, can solve problems such as affecting the stability of milk particles, and achieve the effects of avoiding the increase in the particle size of milk particles, reducing the content of impurities, and avoiding the increase in the particle size of milk particles.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
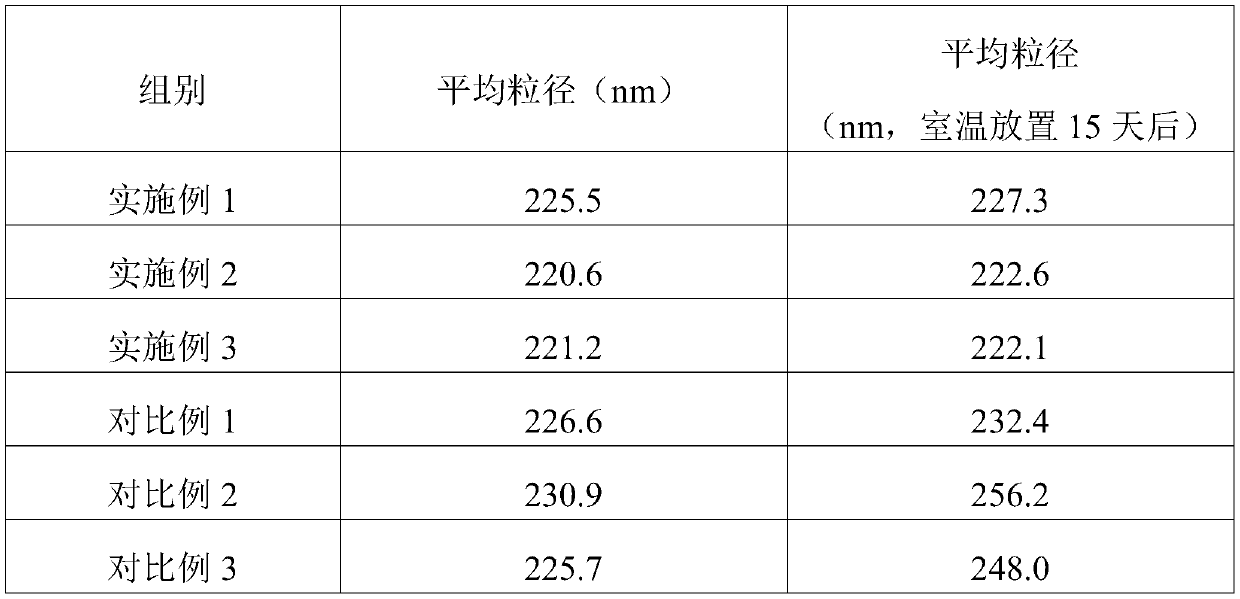
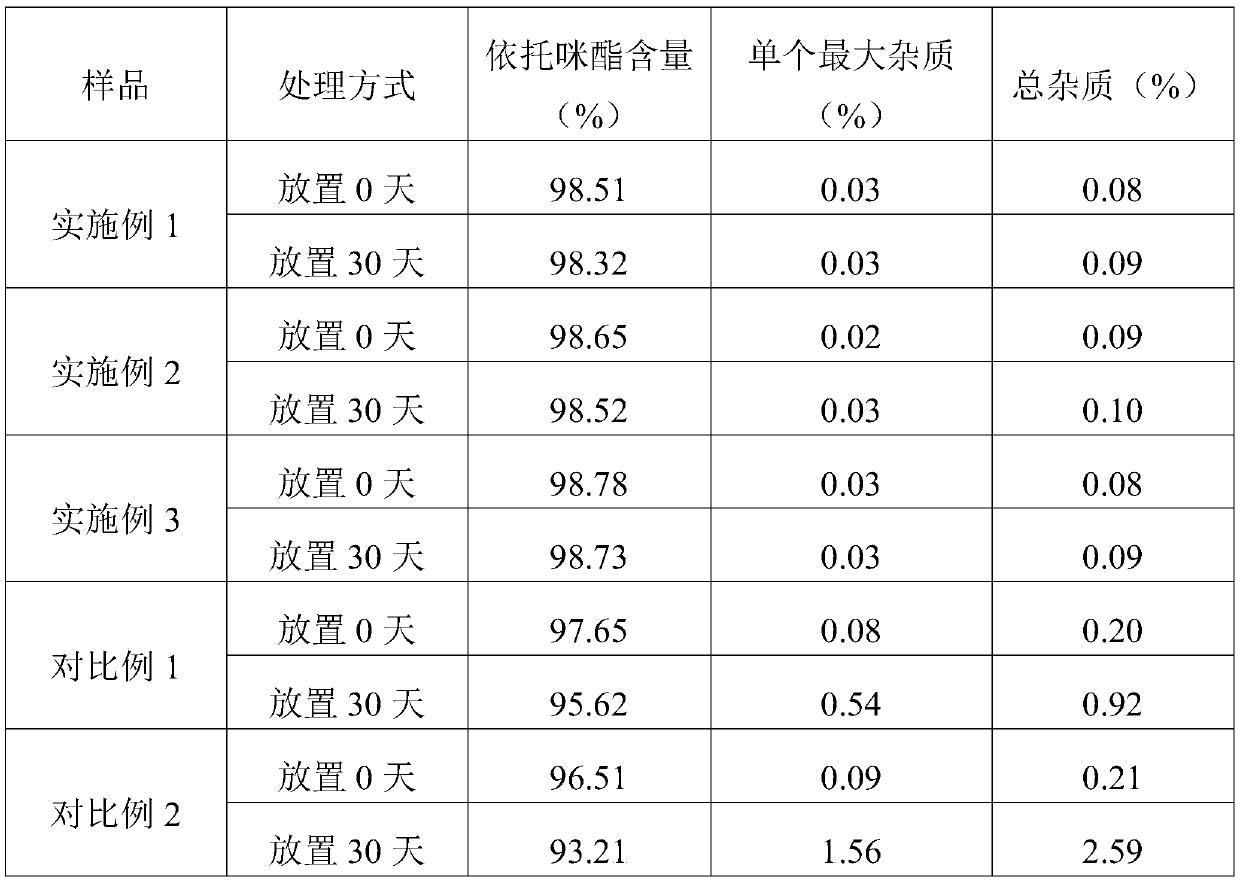
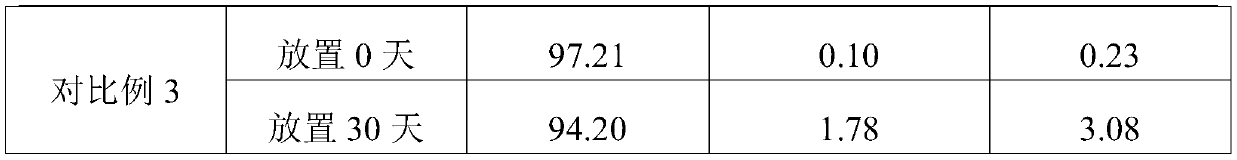
Embodiment 1
[0043] A kind of etomidate injection preparation of embodiment 1
[0044] Its preparation raw materials include by weight:
[0045] Etomidate: 1 part;
[0046] Oil for injection: 40 parts;
[0047] Phospholipids: 4 parts;
[0048] Distearoylphosphatidylglycerol: 4 parts;
[0049] Trehalose: 0.5 parts;
[0050] Mannitol: 4 parts;
[0051] Sodium oleate: 0.4 parts;
[0052] Water for injection: 420 parts.
[0053] The phospholipid is a mixture of hydrogenated soybean lecithin and egg yolk lecithin; the mass ratio of the hydrogenated soybean lecithin and egg yolk lecithin is 1:3.
[0054] Its preparation method comprises the following steps:
[0055] (1) Heat the oil for injection in the prescribed amount to 55°C, add etomidate, phospholipids and distearoylphosphatidylglycerol in the prescribed amount, mix well, and cool to room temperature to obtain oil phase A;
[0056] (2) After heating the water for injection in the prescribed amount to 70°C, add the trehalose, manni...
Embodiment 2
[0061] A kind of etomidate injection preparation of embodiment 2
[0062] Its preparation raw materials include by weight:
[0063] Etomidate: 3 parts;
[0064] Oil for injection: 50 parts;
[0065] Phospholipids: 2 parts;
[0066] Distearoylphosphatidylglycerol: 1 part;
[0067] Trehalose: 1 part;
[0068] Mannitol: 1 part;
[0069] Sodium oleate: 0.05 parts;
[0070] Water for injection: 450 parts.
[0071] The phospholipid is a mixture of hydrogenated soybean lecithin and egg yolk lecithin; the mass ratio of the hydrogenated soybean lecithin and egg yolk lecithin is 1:5.
[0072] Its preparation method comprises the following steps:
[0073] (1) After heating the oil for injection in the prescribed amount to 60°C, add etomidate, phospholipids and distearoylphosphatidylglycerol in the prescribed amount, mix evenly, and cool to room temperature to obtain oil phase A;
[0074](2) After heating the water for injection in the prescribed amount to 65°C, add the trehalose...
Embodiment 3
[0079] A kind of etomidate injection preparation of embodiment 3
[0080] Its preparation raw materials include by weight:
[0081] Etomidate: 1.8 parts;
[0082] Oil for injection: 45 parts;
[0083] Phospholipids: 4 parts;
[0084] Distearoylphosphatidylglycerol: 2 parts;
[0085] Trehalose: 0.7 parts;
[0086] Mannitol: 2 parts;
[0087] Sodium oleate: 0.2 parts;
[0088] Water for injection: 435 parts.
[0089] The phospholipid is a mixture of hydrogenated soybean lecithin and egg yolk lecithin; the mass ratio of the hydrogenated soybean lecithin and egg yolk lecithin is 1:4.
[0090] Its preparation method comprises the following steps:
[0091] (1) After heating the oil for injection in the prescribed amount to 63°C, add etomidate, phospholipids and distearoylphosphatidylglycerol in the prescribed amount, mix well, and cool to room temperature to obtain oil phase A;
[0092] (2) After heating the water for injection in the prescribed amount to 68°C, add trehalos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com