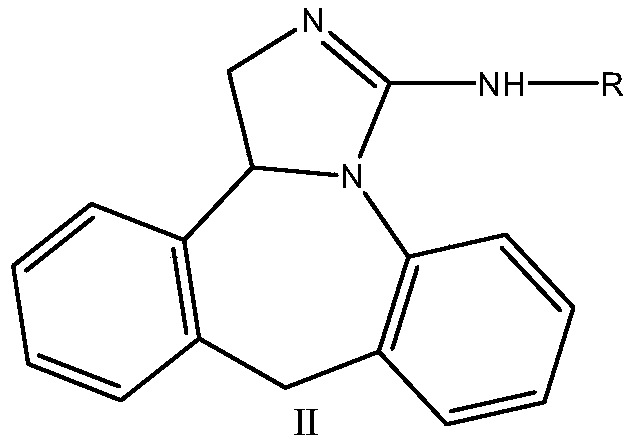
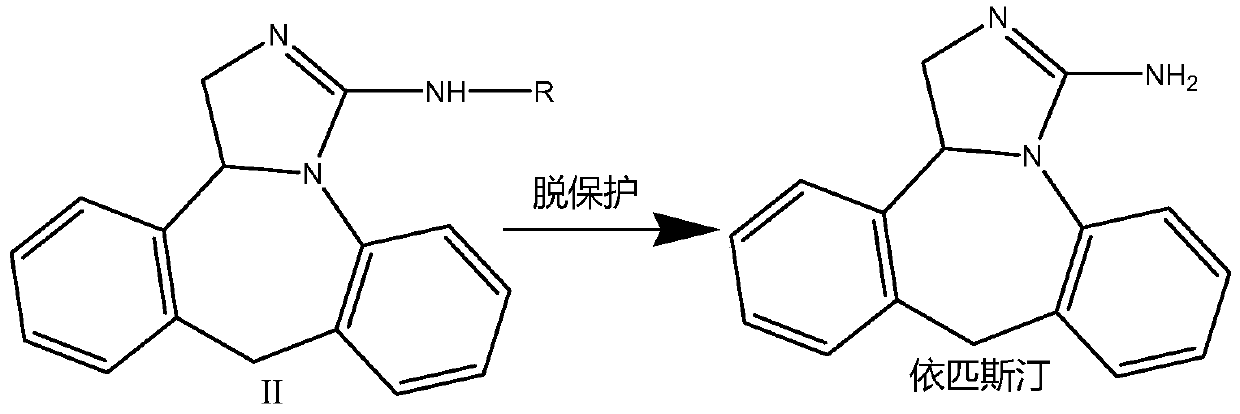
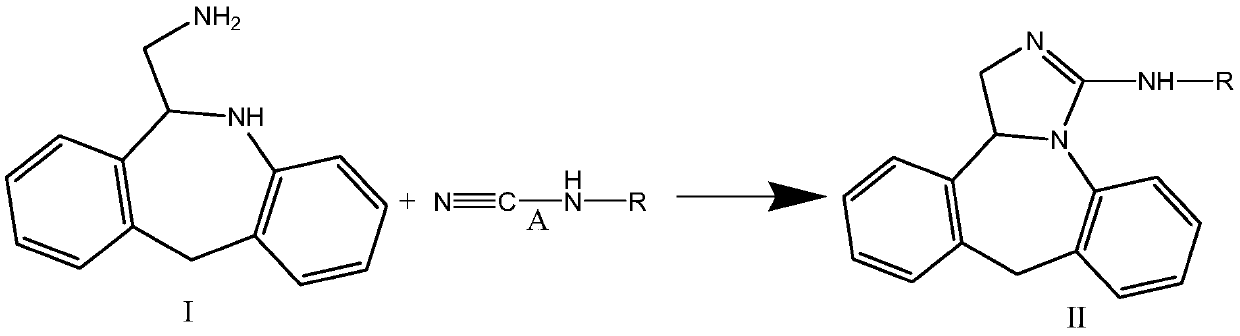
Epinastine hydrochloride intermediate and synthesis method thereof
A synthesis method and epilastine technology are applied in the field of epilastine hydrochloride intermediate and its synthesis, which can solve the problems of not meeting environmental protection and safety production requirements, threatening operator safety, odorous hydrogen sulfide gas, etc. Conducive to safe production and environmental protection, improve safety, and ensure the effect of drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1N-methoxycarbonyl-3-amino-9,13b-dihydro-1H-dibenzo[c,f]imidazo[1,5-a]azepine (formula II-1 compound) synthesis
[0041] The reaction scheme is as follows:
[0042]
[0043] In the above method, the solvent used is water, lower alcohols with 1-4 carbon atoms (methanol, ethanol, n-propanol, isopropanol, n-butanol, etc.), N,N-dimethylformamide, N, One or more of N-dimethylacetamide, tetrahydrofuran, methyltetrahydrofuran, acetone, and acetonitrile.
[0044] Studies have found that in the above method, the pH and reaction temperature of the reaction solution have a very important impact on the reaction. Once the control is not good, the reaction will hardly occur or the by-products will increase significantly. Preferably, the pH of the reaction solution of the present invention is 2-5 (more preferably 2.5-4), and the reaction temperature is 40°C-100°C (more preferably 60°C-90°C).
[0045] The specific operation is:
[0046] 6-Aminomethyl-6,11-dihydro-5H-diben...
Embodiment 2
[0047] Example 2N-tert-butoxycarbonyl-3-amino-9,13b-dihydro-1H-dibenzo[c,f]imidazo[1,5-a]azepine (compound of formula II-2) synthesis
[0048] The reaction scheme is as follows:
[0049]
[0050]Reference Example 1, 6-Aminomethyl-6,11-dihydro-5H-dibenzo[b,e]azepine (26.5g) with 400ml of tert-butanol as solvent, add tert-butyl cyanocarbamate (31.5g), heated to reflux for 3h, cooled, and distilled to dryness to obtain a light brown oily substance, namely N-tert-butoxycarbonyl-3-amino-9,13b-dihydro-1H-dibenzo[c,f ]imidazo[1,5-a]azepine 25.8g, purity 96.7%, yield 87%. m / z 350[M+1] + .
Embodiment 3
[0051] Synthesis of Example 3N-benzyloxycarbonyl-3-amino-9,13b-dihydro-1H-dibenzo[c,f]imidazo[1,5-a]azepine (compound of formula II-3)
[0052] The reaction scheme is as follows:
[0053]
[0054] Referring to Example 1, 6-aminomethyl-6,11-dihydro-5H-dibenzo[b,e]azepine (20.8g) was used as solvent in 400ml of tetrahydrofuran, and benzyl cyanocarbamate (22.5g ), heated to reflux for 10h, cooled, and distilled to dryness to obtain a light brown oily substance, namely N-benzyloxycarbonyl-3-amino-9,13b-dihydro-1H-dibenzo[c,f]imidazo[ 1,5-a]azepine 29.2g, purity 97.7%, yield 82%; m / z 384[M+1] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com