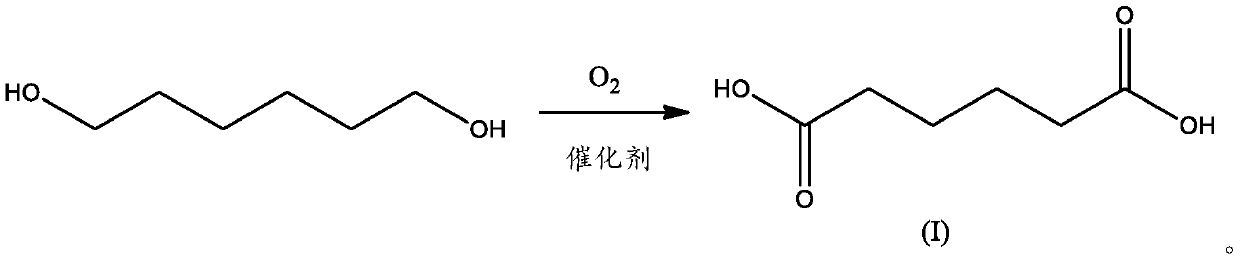
Process for production of adipic acid from 1,6-hexanediol
A technology of adipic acid and hexanediol, applied in the field of novel heterogeneous catalysts, can solve problems such as examples of undisclosed yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
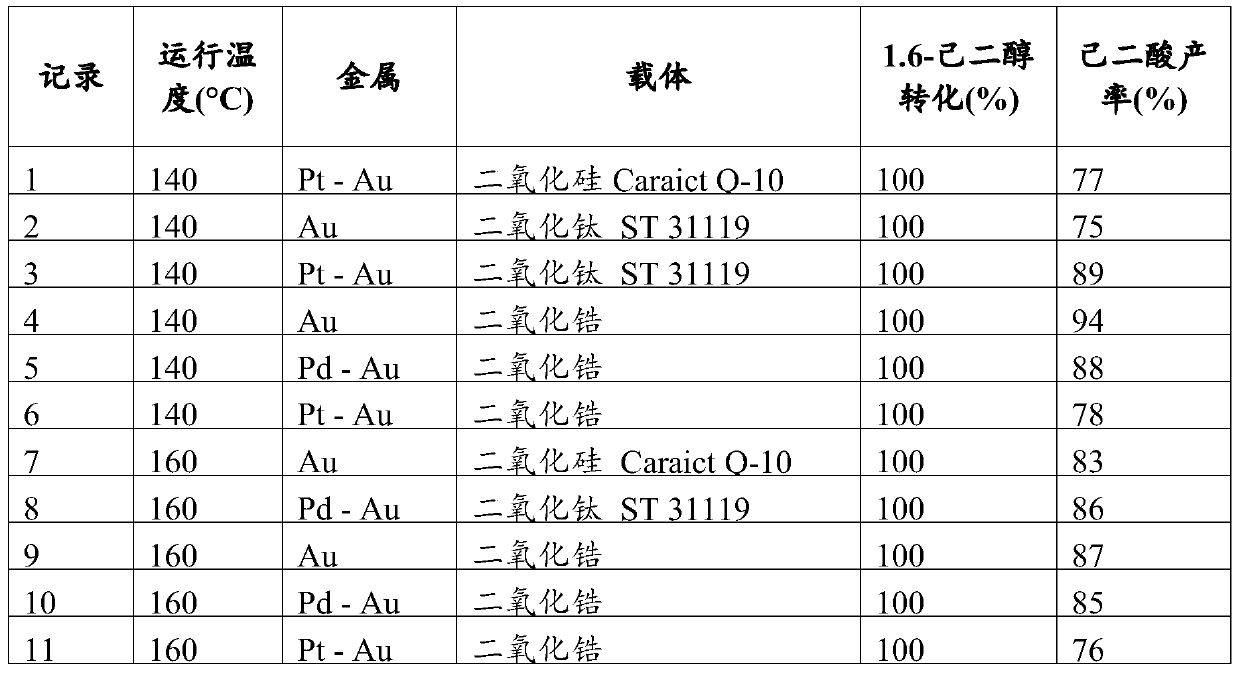
[0063] Example 1: Conversion of 1,6-hexanediol to adipic acid using Au, Au-Pd and Au-Pt catalysts
[0064] About 25 μl of HAuCl4 aqueous solution (comprising 28.6 wt% gold) was added to a suspension containing 600 mg of supports: silica Cariact Q-10 (Fuji Silysia); titanium dioxide ST 31119 (St. Gobain); and zirconium dioxide (Di Zirconia (XZO 1247 from St. Gobain, zirconium hydroxide from MEL Chemicals) was in deionized water (35ml) while shaking. The suspension was shaken for 5 min at room temperature. 1.92 ml of NH4OH (15.85M) aqueous solution was added to the above suspension, and the resulting suspension was shaken at room temperature for 2 hours. Then, the resulting suspension was centrifuged, and the colorless supernatant was decanted. After removing the residual liquid with filter paper, the orange-yellow solid was dried overnight in an oven at 60° C. under dry air.
[0065] The above support containing about 2 wt% Au was equally divided into thirds. Appropriate c...
Embodiment 2
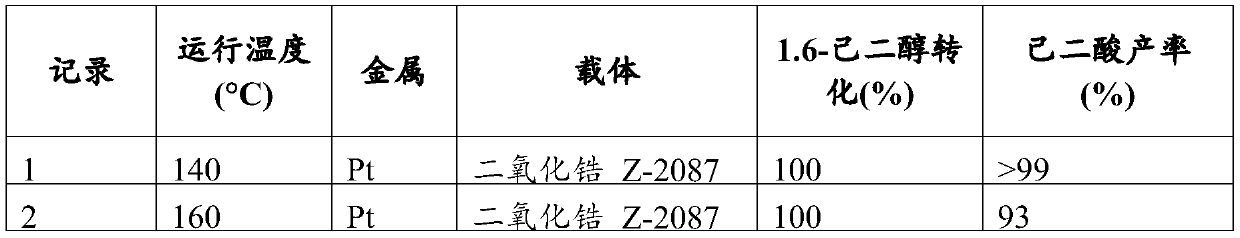
[0069] Example 2: Conversion of 1,6-hexanediol to adipic acid using a Pt catalyst
[0070] The zirconia Z-2087 (Daiichi Kigenso Kagaku Kogyo) support was dried overnight in an oven at 60°C under a dry air purge. The samples were calcined at 650°C for 3 hours under an air atmosphere with a temperature ramp rate of 5°C / min. Appropriate concentration of Pt(NO 3 ) 2 The aqueous solution was added to 200 mg of the carrier and stirred to impregnate the carrier. The samples were dried overnight in an oven at 60 °C under a dry air purge. In forming gas (5% H 2 and 95%N 2 ) atmosphere, the sample was reduced at 350°C for 3 hours, and the temperature ramp rate was 5°C / min. The final catalyst consisted of approximately 3.9 wt% Pt.
[0071] The following assay protocol was employed to proceed to the oxidation of 1,6-hexanediol. The catalyst (approximately 10 mg) was weighed into a glass vial insert, then aqueous 1,6-hexanediol (200 μl, 0.1 M) was added. The glass vial inserts w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| aperture size | aaaaa | aaaaa |
| aperture size | aaaaa | aaaaa |
| aperture size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com