Zwitterionic hyperbranched polyether hydrogel with high stain resistance as well as preparation method and application thereof
A technology of hyperbranched polyether and zwitterions, applied in medical science, tissue regeneration, prosthesis, etc., can solve the problems of insufficient antifouling performance and poor stability of hydrogel, and achieve excellent antifouling performance and excellent biocompatibility Performance and stability, the effect of meeting the needs of long-term stability and biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A preparation method of zwitterionic hyperbranched polyethylene glycol hydrogel with high antifouling performance, comprising the following steps:
[0033] (1) Synthesis of double bond-containing carboxybetaine zwitterionic monomer (CBMA)
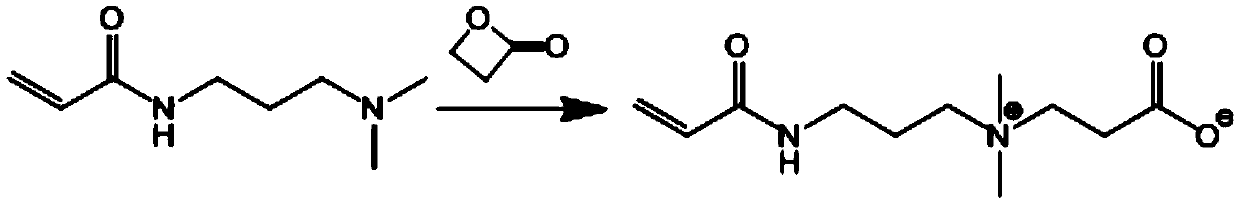
[0034] Dissolve N-(N,N'-dimethylaminopropyl)acrylamide (DMAPAA, 4.0g, 25.6mmol) in 10mL of anhydrous acetone, and then add β-propiolactone monomer (2g, 27.8mmol) Dissolve it in 10mL of anhydrous acetone, and slowly add it dropwise to the acetone solution of DMAPAA under the stirring condition of 500r / min, and continue to react at room temperature for 24h under the protection of nitrogen. After washing three times and drying in vacuum, the double bond-containing zwitterionic monomer CBMA was obtained, and its chemical reaction formula is shown below.
[0035]
[0036] (2) Synthesis of functional modified multi-arm polyethylene glycol
[0037] Mix eight-armed polyethylene glycol (8sPEG, Mw=20k) with thiourea and ethyl 2-bromometha...
Embodiment 2
[0049] A preparation method of zwitterionic hyperbranched polyethylene glycol hydrogel with high antifouling performance, comprising the following steps:
[0050] (1) Synthesis of double bond-containing sulfobetaine zwitterionic monomer (SBMA)
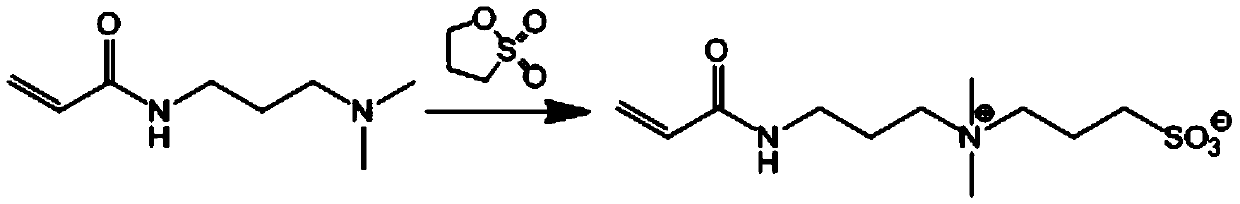
[0051] Dissolve N-(N,N'-dimethylaminopropyl)acrylamide (DMAPAA, 2.0g, 12.8mmol) in 10mL of anhydrous acetone, and then add β-sultone monomer (1g, 14.02mmol ) was dissolved in 10mL of anhydrous acetone, and under the stirring condition of 600r / min, it was slowly added dropwise to the acetone solution of DMAPAA, and the reaction at room temperature was continued for 18h under the protection of nitrogen. Washed with acetone three times and dried in vacuum to obtain double bond-containing zwitterionic monomer SBMA, the chemical reaction formula of which is shown below.
[0052]
[0053] (2) Synthesis of functionalized modified multi-arm polyethylene glycol
[0054]Mix eight-armed polyethylene glycol (8sPEG, Mw=10k) with thiourea and e...
Embodiment 3
[0066] A preparation method of zwitterionic hyperbranched polyethylene glycol hydrogel with high antifouling performance, comprising the following steps:
[0067] (1) Synthesis of double bond-containing carboxybetaine zwitterionic monomer (CBMA)
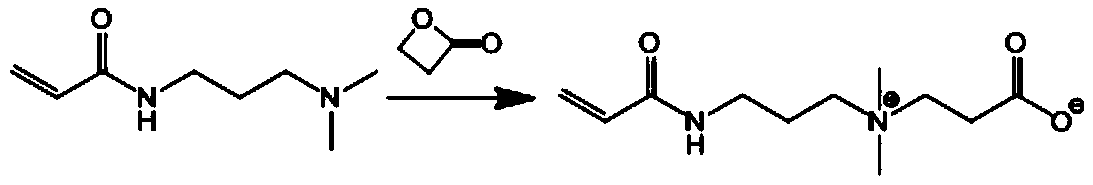
[0068] N-(N,N'-dimethylaminopropyl)acrylamide (DMAPAA, 4.0g, 25.6mmol) was dissolved in 15mL of anhydrous acetone, and then β-propiolactone monomer (1.5g, 20.4mmol ) was dissolved in 15mL of anhydrous acetone, and under the stirring condition of 800r / min, it was slowly added dropwise to the acetone solution of DMAPAA, and the reaction at room temperature was continued for 20h under nitrogen protection. After the reaction was completed, the white precipitate was obtained by filtration, and used Washed with acetone three times and dried in vacuum to obtain double bond-containing zwitterionic monomer CBMA, its chemical reaction formula is shown below.
[0069]
[0070] (2) Synthesis of functionalized modified multi-arm polyethylene ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com