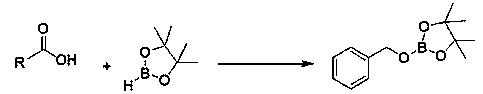Application of n-butyllithium in catalysis of hydroboration reaction of aliphatic carboxylic acid and borane
一种脂肪族羧酸、正丁基锂的技术,应用在催化反应、物理/化学过程催化剂、有机化合物/氢化物/配位配合物催化剂等方向,能够解决成本高等问题,达到反应时间短、收率高、反应条件温和的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: N-butyllithium catalyzes the hydroboration reaction of acetic acid and pinacol borane
[0024] Under an inert gas atmosphere, add acetic acid (28.6 μL, 0.5 mmol) to the reaction flask after dehydration and deoxygenation treatment, add pinacol borane (290 μL, 2 mmol) with a pipette gun, and finally add 10 μL n-butyl Lithium tetrahydrofuran solution (0.1M) (0.2 mol% dosage, the same below), reacted at room temperature for 15 minutes, exposed the reaction solution to air, and removed the solvent to obtain the product boric acid ester, which was obtained by s-trimethoxybenzene (84.08 mg , 0.5 mmol) as internal standard, with CDCl 3 Dissolved, stirred for 10 minutes, sampled, and NMR. Calculated 1 H yield was 99%. NMR data of the product: 1 H NMR (400 MHz, CDCl 3 ): δ3.88 (q, 2H, CH 2 ), 1.25 (s, 36H, CH 3 ), 1.21 (br s, 3H, CH 3 ).
[0025] When pinacol borane (218 μL, 1.5 mmol), the yield was 95%; when pinacol borane (363 μL, 2.5 mmol), the yield was...
Embodiment 2
[0026] Example 2: n-Butyl Lithium Catalyzed Hydroboration Reaction of Valeric Acid and Pinacol Borane
[0027] Under an inert gas atmosphere, add valeric acid (54.38 μL, 0.5 mmol) to the reaction flask after dehydration and deoxygenation treatment, add pinacol borane (290 μL, 2 mmol) with a pipette gun, and finally add n-butyl The tetrahydrofuran solution of lithium (0.2 mol% dosage) was reacted at room temperature for 15 minutes, the reaction solution was exposed to air, and the solvent was removed to obtain the product borate, with s-trimethoxybenzene (84.12 mg, 0.5 mmol) as internal standard, with CDCl 3 Dissolved, stirred for 10 minutes, sampled, and NMR. Calculated 1 H yield was 92%. NMR data of the product: 1 H NMR (400 MHz, CDCl 3 ): δ 3.81 (t, 2H,OCH 2 ), 1.52-1.56 (m, 2H,CH 2 ), 1.30-1.52 (m, 4H, CH 2 ), 1.28(s, 36H, CH), 0.86(t, 3H, CH 3 ).
Embodiment 3
[0028] Embodiment 3: n-Butyllithium catalyzes the hydroboration reaction of hexanoic acid and pinacol borane
[0029] Under an inert gas atmosphere, add hexanoic acid (62.52 μL, 0.5 mmol) to the reaction flask after dehydration and deoxygenation treatment, add pinacol borane (290 μL, 2 mmol) with a pipette gun, and finally add n-butyl The tetrahydrofuran solution of lithium (0.2 mol% dosage) was reacted at room temperature for 15 minutes, the reaction solution was exposed to air, and the solvent was removed to obtain the product borate, with s-trimethoxybenzene (84.01 mg, 0.5 mmol) as the internal standard, with CDCl 3 Dissolved, stirred for 10 minutes, sampled, and NMR. Calculated 1 H yield was 90%. NMR data of the product: 1 H NMR (400 MHz, CDCl 3 ): δ 3.76 (t, 2H,OCH 2 ), 1.46-1.52 (m, 2H,CH 2 ), 1.24-1.35 (m, 6H, CH 2 ), 1.19 (s, 48H, CH 3 ), 0.82(t, 3H, CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com