Human interferon alpha receptor binding related site mutant and application thereof
An interferon alpha, receptor binding technology, applied in the fields of medicine and bioengineering, can solve the problems of different sensitivities, differences, non-conservation, etc., and achieve the effects of strong activation effect, high induction effect, and strong activation effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
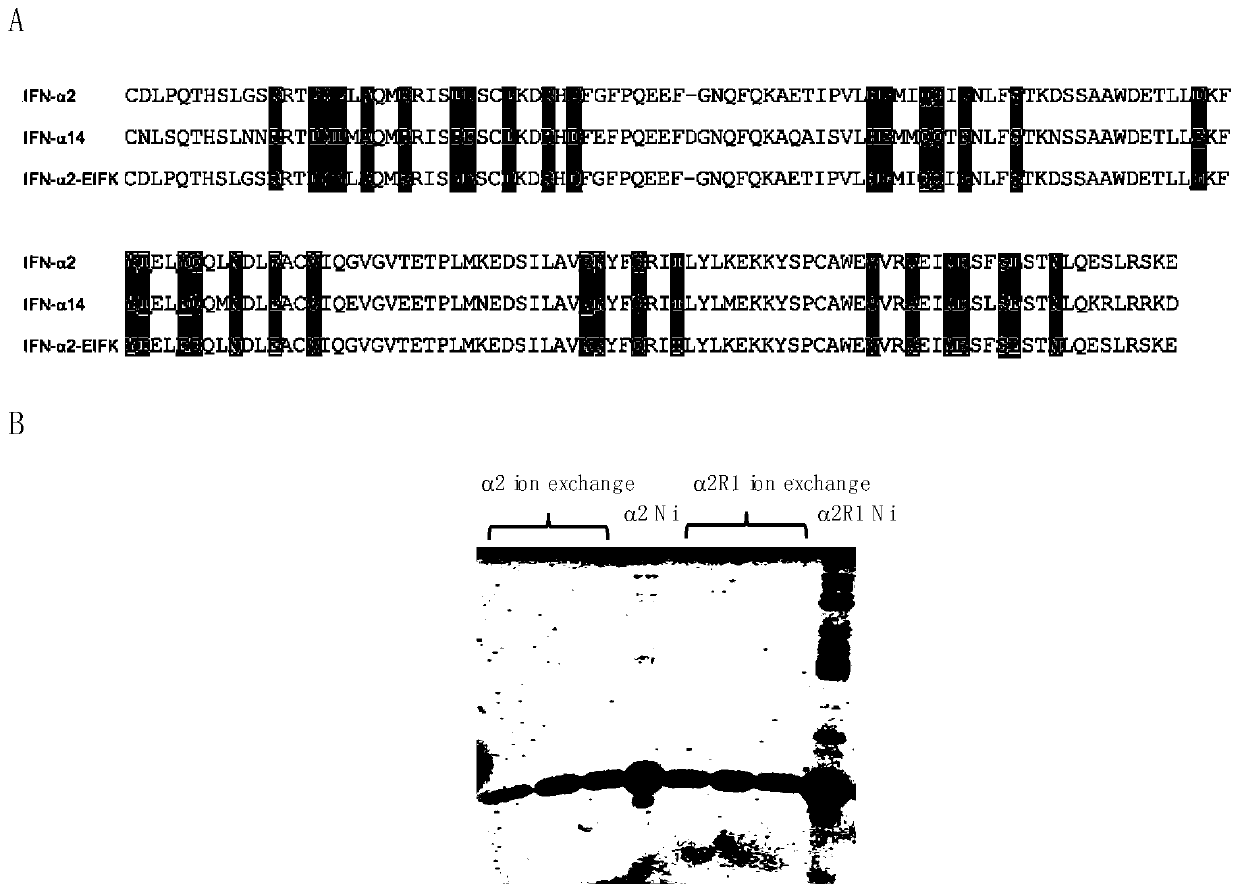
[0025] Example 1 Preparation and Purification of Human IFN-α2 and IFN-α2-EIFK
[0026] Human IFN-α2 gene coding sequence (SEQ ID No.1), IFN-α2-EIFK (SEQ ID NO.2) interferon mutant sequence (such as figure 1 Shown in A), and the IFN-α14 sequence (SEQ ID No.3) is cloned on the prokaryotic expression vector, followed by prokaryotic expression of the recombinant protein;
[0027] (1) Transform the recombinant plasmid into E.coli BL-21, spread it on solid LB medium containing interferon, wait at 37°C for 16 hours, pick a single colony to 3-4ml LB and shake it overnight. Introduce the bacteria into the shaker at a ratio of 1:100. When the OD600 reading is between 0.5-0.6, induce the final concentration of IPTG at 10 μM, and express at 16°C for 20 hours. After the protein induction is completed, add the bacterial solution to 50ml Centrifuge tube, 5000g, centrifuge at 4°C for 10min, discard the supernatant;
[0028] (2) The bacteria after centrifugation are resuspended with buffer A...
Embodiment 2
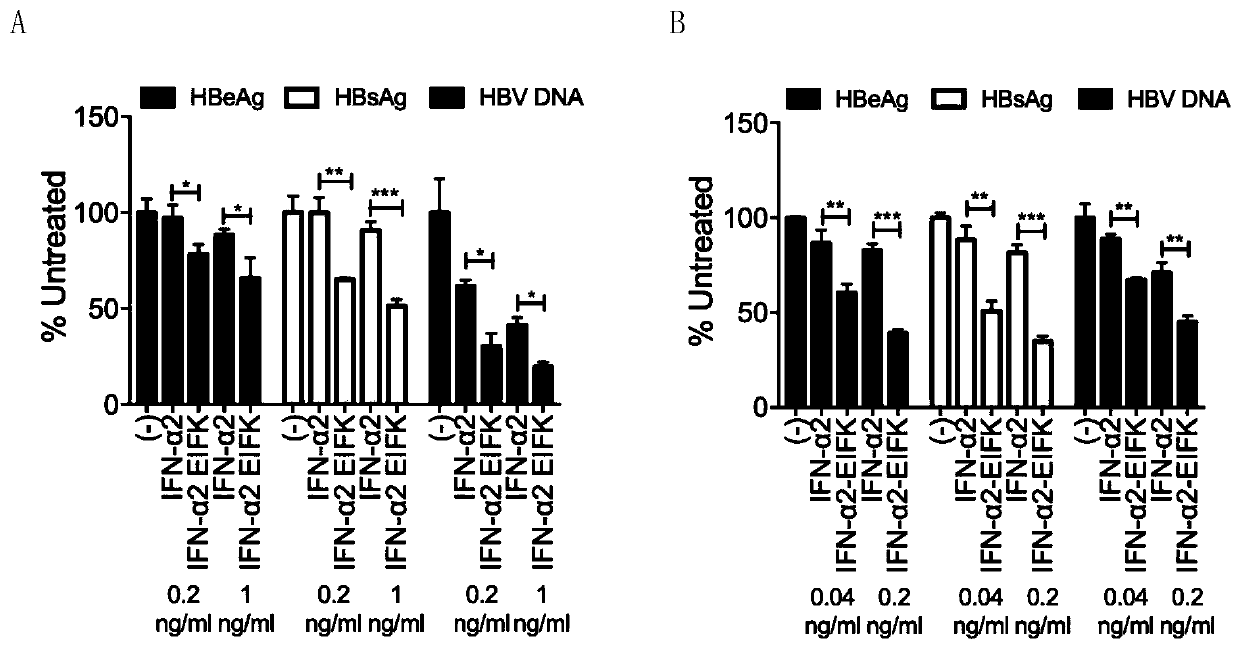
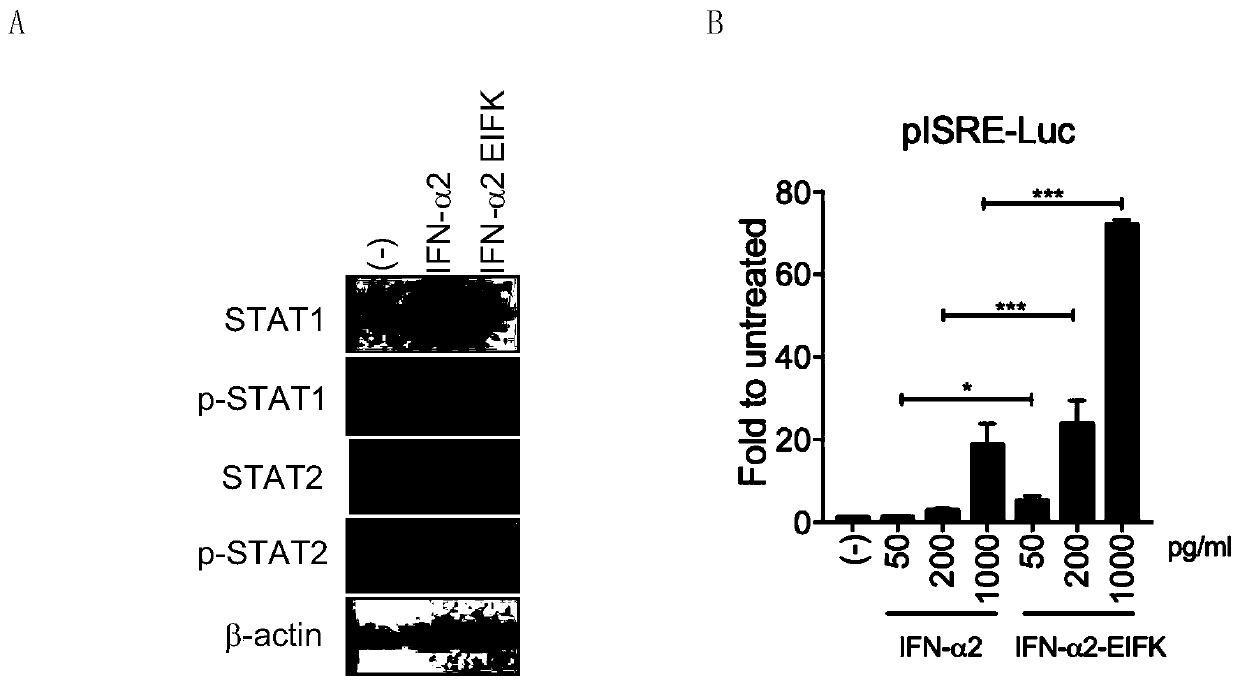
[0035] Embodiment 2 HBV infection system
[0036] The HepG2-NTCP ( figure 2 A) or PHH ( figure 2 B) Cells, the production of hepatitis B virus e antigen (HBeAg) and DNA are inhibited to varying degrees:
[0037] (1) Culture of HepG2-NTCP cells: DMEM culture medium (Gibco company, plus 10% fetal bovine serum, 100U / ml penicillin, 100mg / ml streptomycin) was used for ordinary culture at 37°C under 5% CO2 saturated water vapor environment Constant temperature culture. When carrying out hepatitis B virus infection experiments, the medium for infection: ordinary medium + 2.5% DMSO, the culture of PHH cells: purchased from Shanghai Ruide Biology and cultured with special commercial medium;
[0038] (2) The hepatitis B virus used for infection was purified by our laboratory. After the HepAD38 supernatant was collected, the supernatant was concentrated about 100 times by PEG8000 precipitation method, and the infection was performed at a concentration of 200 copies / cell during infe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com