Fmoc-protected amino acid purity and related substance analysis method
A related substance and analysis method technology, applied in the field of Fmoc-protected amino acid purity related substance analysis and detection, can solve the problems of irreversible damage to the chromatographic column, low accuracy and practicability of the test method, shortened service life of the chromatographic column, etc. Effects of sample decomposition or instrument corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
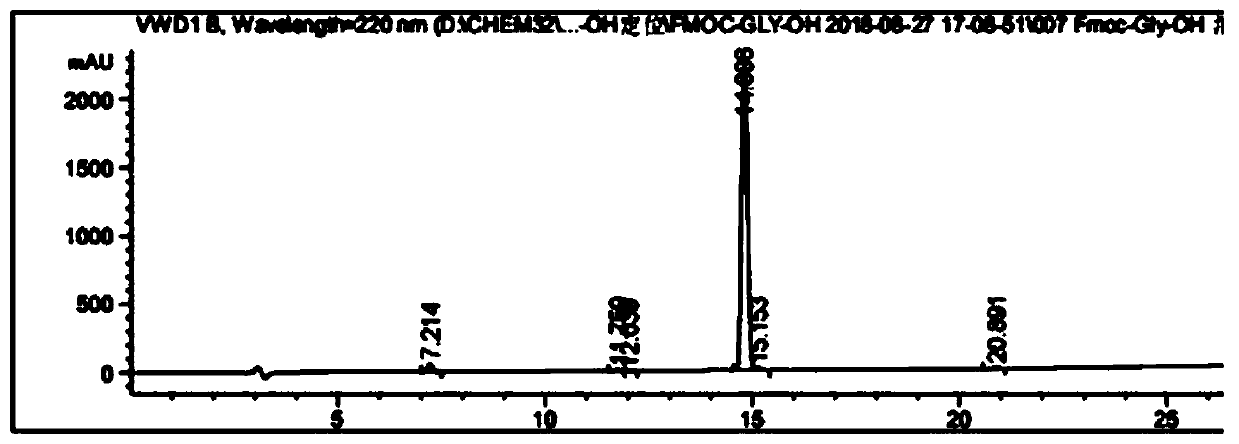
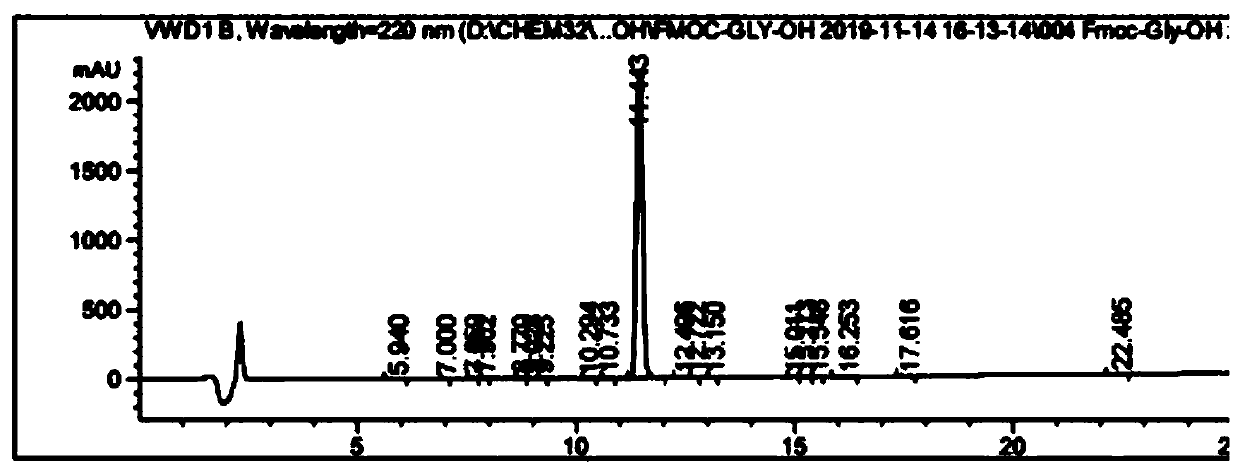
[0049] A method for analyzing the purity of Fmoc-Gly-OH and related substances, comprising the following steps:
[0050] S1. Set chromatographic conditions
[0051] Instrument: high performance liquid chromatography
[0052] Column: C 18 5μm 4.6×250mm
[0053] Detector: UV, 220-260nm
[0054] Flow rate: 0.5-1.0ml / min
[0055] Column temperature: 20-30°C
[0056] Injection volume: 5-15ul
[0057] Gradient elution conditions:
[0058]
[0059] S2, prepare mobile phase
[0060] Mobile phase A: trifluoroacetic acid: water = 0.2-1: 1000; mobile phase B: trifluoroacetic acid: acetonitrile = 0.2-1: 1000;
[0061] S3, prepare Fmoc-Osu impurity control solution
[0062] Accurately weigh the relevant impurity Fmoc-Osu standard, put it in a volumetric flask, prepare a 0.5-2.0% impurity solution with acetonitrile, shake well for later use, accurately weigh the Fmoc-Gly-OH standard, and put it in a volumetric flask , dissolved in acetonitrile, and then added impurity solution...
Embodiment 2
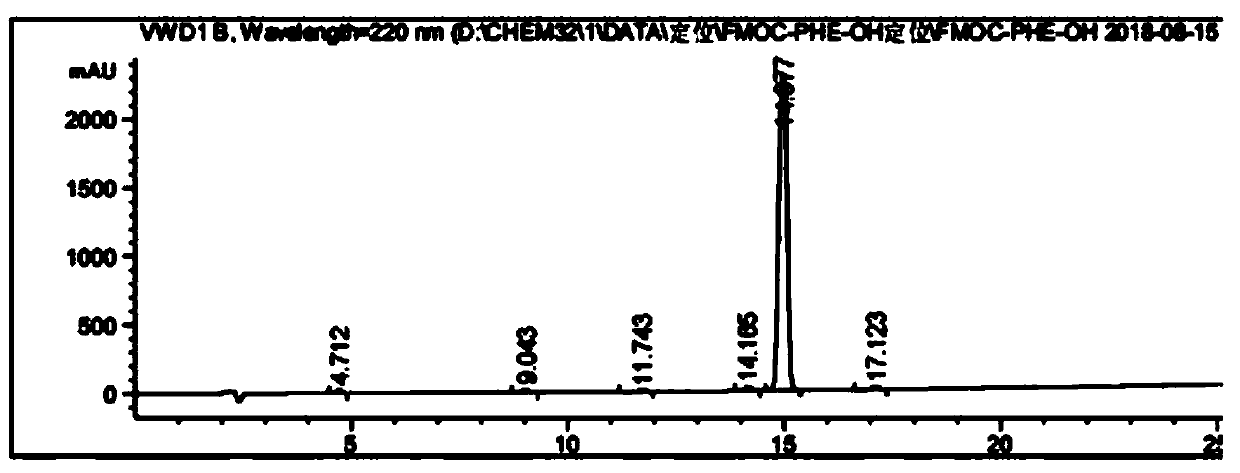
[0089] A method for analyzing Fmoc-Phe-OH purity and related substances, comprising the following steps:
[0090] S1. Set chromatographic conditions
[0091] Instrument: high performance liquid chromatography
[0092] Column: C 18 5μm 4.6×250mm
[0093] Detector: UV, 220-260nm
[0094] Flow rate: 0.5-1.2ml / min
[0095] Column temperature: 20-30°C
[0096] Injection volume: 5-20ul
[0097] Gradient elution conditions:
[0098]
[0099] S2, prepare mobile phase
[0100] Mobile phase A: trifluoroacetic acid: water = 0.2-1: 1000; mobile phase B: trifluoroacetic acid: acetonitrile = 0.2-1: 1000;
[0101] S3, prepare Fmoc-Osu impurity control solution
[0102] Accurately weigh the relevant impurity Fmoc-Osu standard, put it in a volumetric flask, prepare a 0.5-2.0% impurity solution with acetonitrile, shake well for later use, and accurately weigh the Fmoc-Phe-OH standard, and put it in a volumetric flask , dissolved in acetonitrile, and then added impurity solution, s...
Embodiment 3
[0128] A Fmoc-Lys(Boc)-OH purity and related substance analysis method, comprising the following steps:
[0129] S1. Set chromatographic conditions
[0130] Instrument: high performance liquid chromatography
[0131] Column: C 18 5μm 4.6×250mm
[0132] Detector: UV, 220-260nm
[0133] Flow rate: 0.5-1.2ml / min
[0134] Column temperature: 20-30°C
[0135] Injection volume: 5-20ul
[0136] Gradient elution conditions:
[0137]
[0138]
[0139] S2, prepare mobile phase
[0140] Mobile phase A: trifluoroacetic acid: water = 0.2-1: 1000; mobile phase B: trifluoroacetic acid: acetonitrile = 0.2-1: 1000;
[0141] S3, prepare Fmoc-Osu impurity control solution
[0142] Accurately weigh the relevant impurity Fmoc-Osu standard product, place it in a volumetric flask, prepare a 0.5-2.0% impurity solution with acetonitrile, shake it up for later use, and accurately weigh the Fmoc-Lys(Boc)-OH standard product, place it in In the volumetric flask, dissolve with acetonitrile,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com