Application of schisandra polysaccharide in preparation of medicine or health product for treating inflammatory bowel disease
A technology of inflammatory bowel disease and schisandra, applied in the field of biopharmaceuticals and molecular biology, can solve the problems of multiple side effects and insignificant effects, and achieve the effect of improving colitis and inflammatory bowel disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 A preparation method of an extract capable of improving inflammatory bowel disease
[0036] Weigh 400 g of each dried sample of Schisandra chinensis in raw vinegar, reflux three times the amount (1200 mL) of petroleum ether for 4 hours to degrease, and remove the petroleum ether after degreasing. Then add pure water (3200mL) in an amount 8 times the weight of the decoction pieces, heat to reflux at 100°C, extract twice for 3 hours each time, and combine the extracts. Concentrate the water extract to 200mL, add absolute ethanol to make the alcohol content reach 80%, let stand overnight, and centrifuge to obtain the supernatant filtrate and precipitate, which is Schisandra crude polysaccharide.
[0037] Deproteinization by Savage method: dissolve the crude polysaccharide of Schisandra chinensis with pure water to 400mL, because the protein in chloroform can be deformed and precipitated, add chloroform and n-butanol in the ratio of polysaccharide solution:chlorof...
Embodiment 2 5
[0039] Determination of total polysaccharide content in embodiment 2 Schisandra polysaccharide extract
[0040] Take 1 mg of schisandra polysaccharide freeze-dried powder refined in Example 1, add distilled water to make the volume to 10 mL, that is, the concentration of the sample solution is 0.1 mg / mL. Using glucose as a control, the total polysaccharide content was determined at 490nm by phenol-sulfuric acid method and ultraviolet spectrophotometer. According to calculation, the total polysaccharide content in the refined Schisandra polysaccharide extract is 85-95%, as shown in Table 1.
[0041] The polysaccharide content (X±SD, n=3) in each batch of Schisandra chinensis polysaccharide extract sample of table 1
[0042]
[0043]
Embodiment 3 5
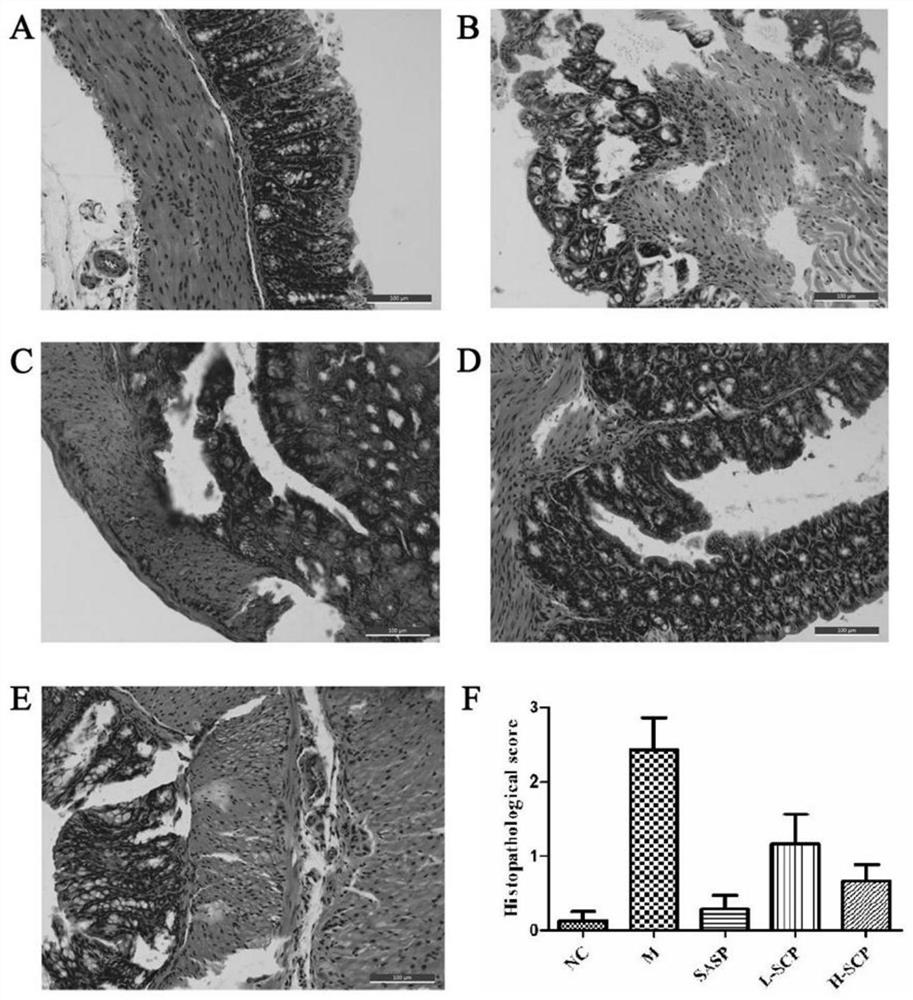
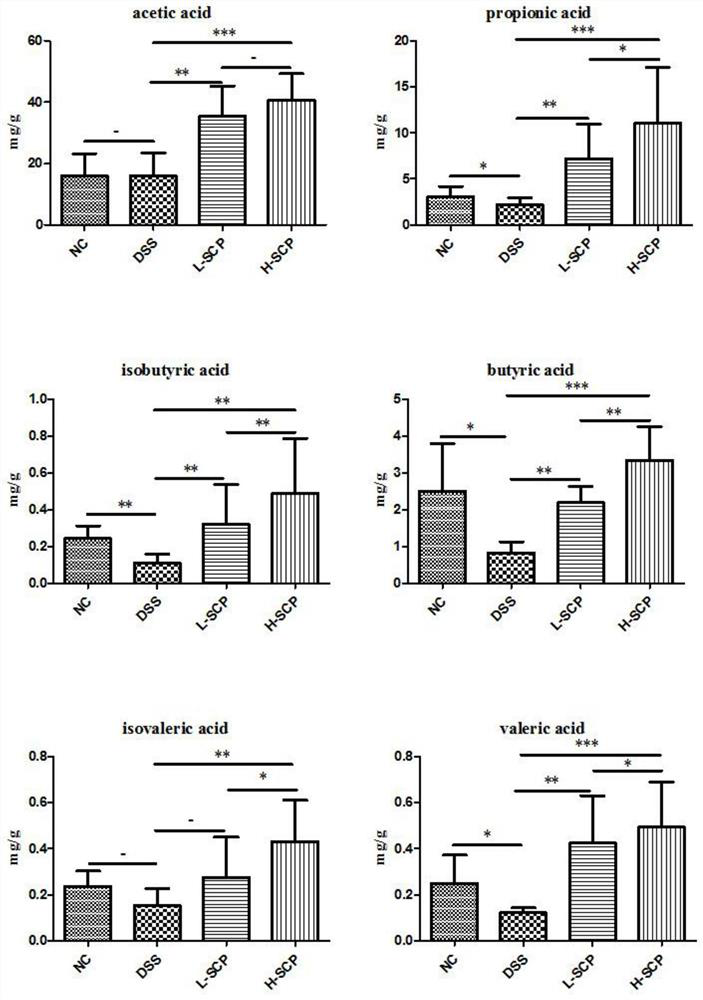
[0044] Example 3 Study on the Effect of Schisandra Polysaccharide Extract on Colitis Mice
[0045] The Schisandra polysaccharide extract was obtained by the extraction method in Example 1, and two extract solutions of different concentrations (ie high and low doses) were prepared, the low dose: 0.2 g / mL, and the high dose: 0.4 g / mL.
[0046] C57BL6 mice, body weight 20.0+2.0g. All animals were fed under (SPF) grade and adaptively fed for one week. DSS was made into 2% DSS and 3.5% DSS aqueous solution with sterilized distilled water, instead of drinking water, and the experimental C57BL6 mice were allowed to drink freely to induce colitis. 2% DSS aqueous solution was given on the 1st-3rd day, and 3.5% DSS aqueous solution was replaced on the 4th-7th day.
[0047] The experimental mice were fed adaptively for one week, and were randomly divided into 8 groups. The normal group (Normal): drank drinking water without DSS for 21 days. Model group (DSS): Mice acquired acute colit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com