Total-nutrient formula food for assisting in regulating intestinal immune functions
A technology of formula food and complete nutrition, which is applied in the direction of food ingredients, food ingredient functions, vitamin-containing food ingredients, etc., to achieve the effect of improving inflammatory bowel disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Design of nutritional formula containing mixed protein
[0049] Prepare nutritional formula food according to the following formula, each 100g contains: carbohydrate (maltodextrin) 60g, protein 18g, fat 12g, dietary fiber 4g, vitamin A 669.6IU, vitamin D 3.125μg, vitamin E 0.0056IU, vitamin K1 0.024 mg, vitamin B1 1.025mg, vitamin B2 1.025mg, vitamin B6 1.25mg, vitamin B120.003mg, vitamin C 85.78mg, niacin 9.125mg, folic acid 0.12mg, calcium pantothenate 5.71mg, D-biotin 0.01mg, chloride Sodium 953.23mg, potassium citrate monohydrate 217.92mg, copper carbonate 1.388mg, magnesium oxide 247.016mg, iron citrate 43.424mg, zinc carbonate 10.304mg, manganese carbonate 3.48mg, calcium carbonate 1005.1mg, potassium dihydrogen phosphate 1466.39mg, Potassium iodate 0.089mg, sodium selenite 0.061mg, chromium potassium sulfate 0.324mg, ammonium molybdate tetrahydrate 0.094mg, choline chloride 167.56mg. Among them, the carbohydrate is maltodextrin, the protein is a mixtu...
Embodiment 2
[0051] Example 2: Potato protein group
[0052] Prepare nutritional formula food according to the following formula, each 100g contains: carbohydrate (maltodextrin) 60g, protein 18g, fat 12g, dietary fiber 4g, vitamin A 669.6IU, vitamin D 3.125μg, vitamin E 0.0056IU, vitamin K1 0.024 mg, vitamin B1 1.025mg, vitamin B2 1.025mg, vitamin B6 1.25mg, vitamin B120.003mg, vitamin C 85.78mg, niacin 9.125mg, folic acid 0.12mg, calcium pantothenate 5.71mg, D-biotin 0.01mg, chloride Sodium 953.23mg, potassium citrate monohydrate 217.92mg, copper carbonate 1.388mg, magnesium oxide 247.016mg, iron citrate 43.424mg, zinc carbonate 10.304mg, manganese carbonate 3.48mg, calcium carbonate 1005.1mg, potassium dihydrogen phosphate 1466.39mg, Potassium iodate 0.089mg, sodium selenite 0.061mg, chromium potassium sulfate 0.324mg, ammonium molybdate tetrahydrate 0.094mg, choline chloride 167.56mg.
[0053] Among them, the protein is potato protein; the carbohydrate is maltodextrin; the mass ratio o...
Embodiment 3
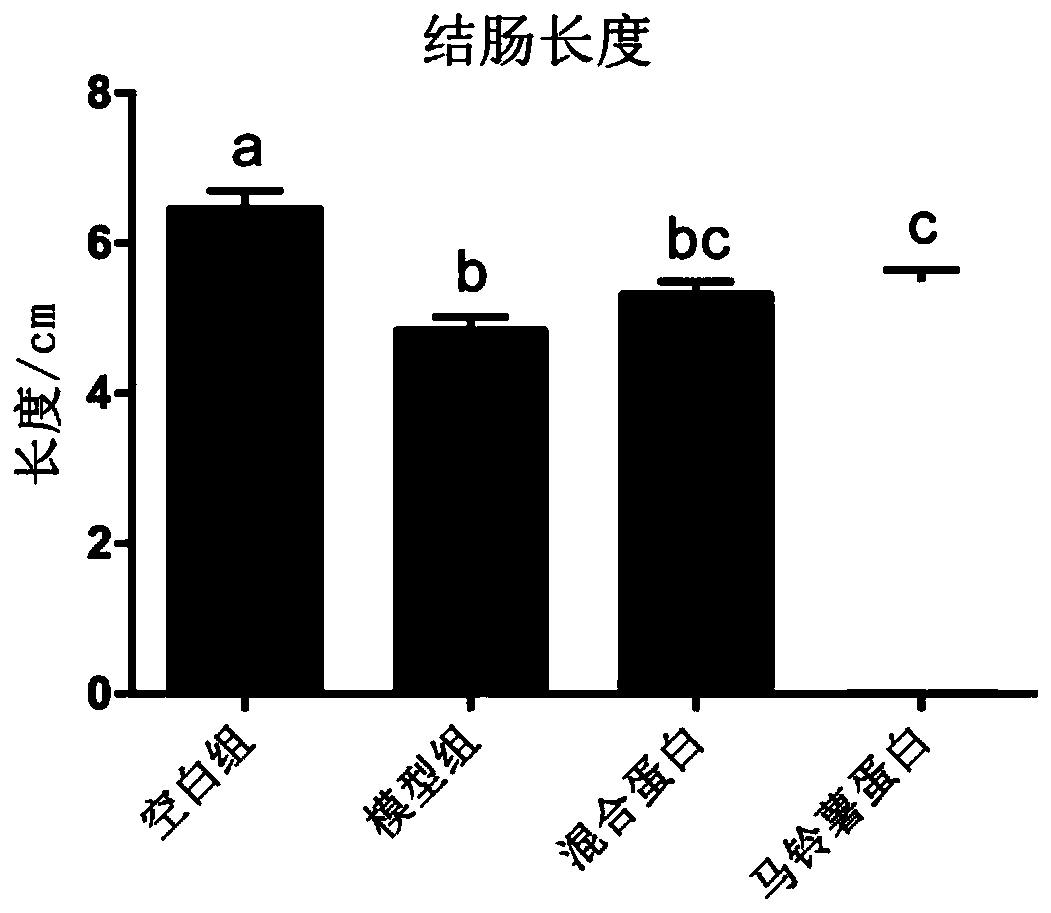
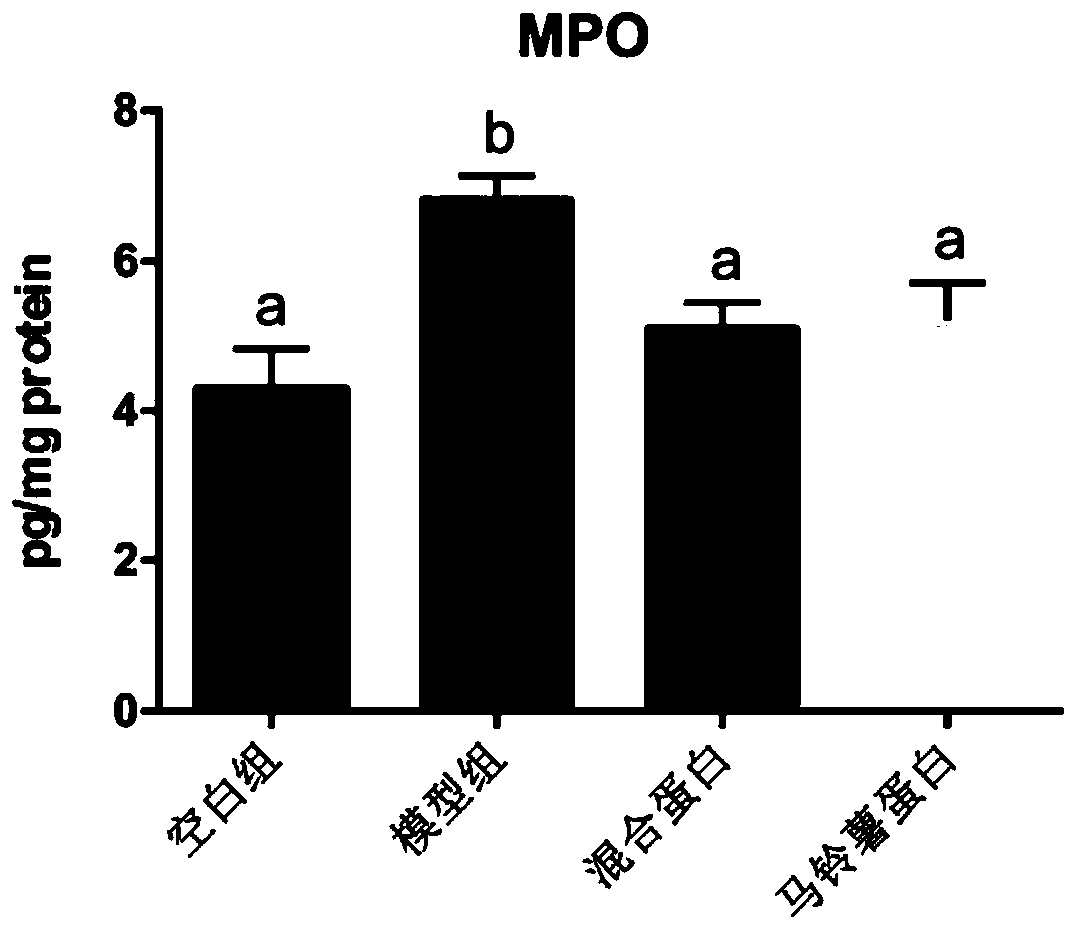
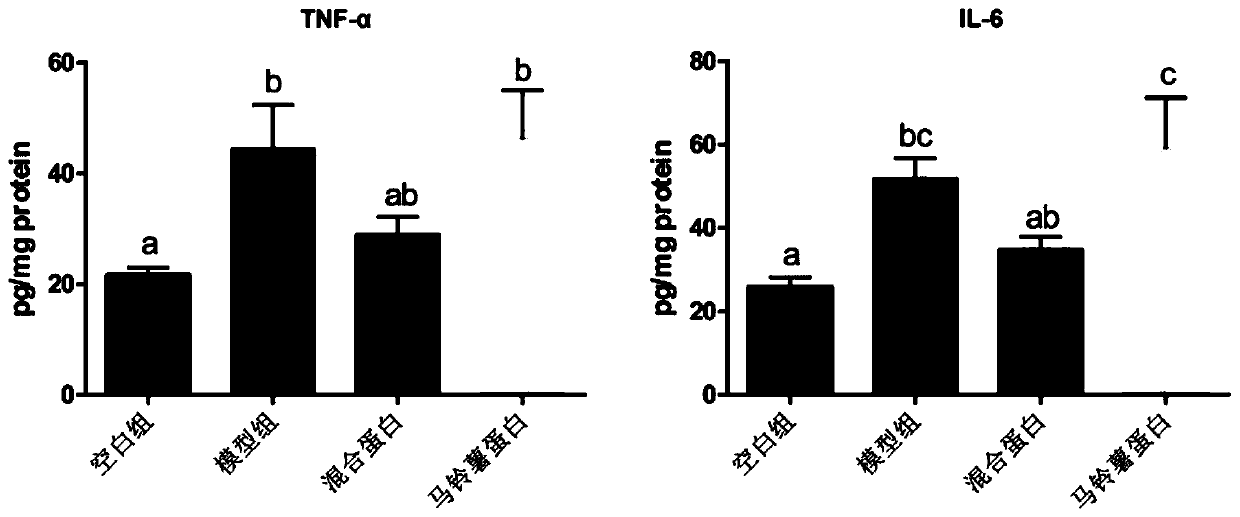
[0055] Example 3 Inflammatory Bowel Disease Whole Nutrition Formula Food Inhibits Colon Shortening in Mice with Enteritis
[0056] 40 healthy male C57BL / 6 mice of SPF grade 18-20g in 6-8 weeks were randomly divided into 4 groups: blank group (feeding basal feed), model group (feeding basal feed), mixed protein group (feeding the basal feed of Example 1) Nutritional formula food), potato protein group (feeding the nutritional formula food of embodiment 2). Ten animals in each group were raised in the Experimental Animal Center of Jiangnan University, with a constant temperature of 21-26°C, humidity of 40-70%, noise less than or equal to 60dB, and animal illumination of 15-20LX. All animal experimental procedures were reviewed and approved by the Animal Welfare and Ethics Management Committee of Jiangnan University.
[0057] From the 1st day to the 7th day of the experiment, 3.5% DSS was added to the drinking water of the mice to induce acute colitis, and they drank 3 cycles (7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com