Kit for detecting gene mutations in acute lymphoblastic leukemia based on fluorescence quantitative PCR and method
An acute lymphocyte and fluorescent quantitative technology, which is applied in the determination/inspection of microorganisms, biochemical equipment and methods, DNA/RNA fragments, etc., can solve the problems of inability to detect large fragments of genome copy number variation, and avoid false positives, Easy operation and high sensitivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
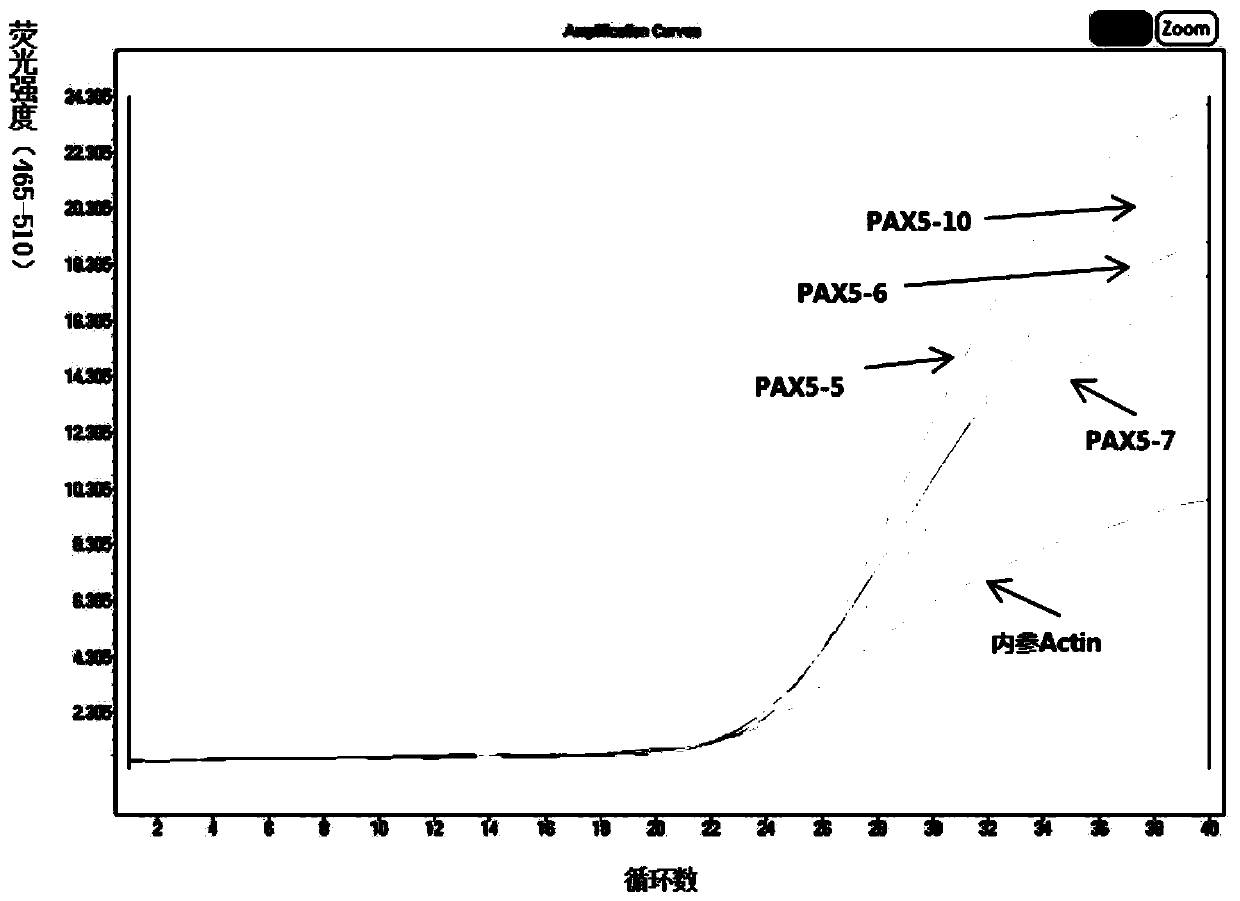
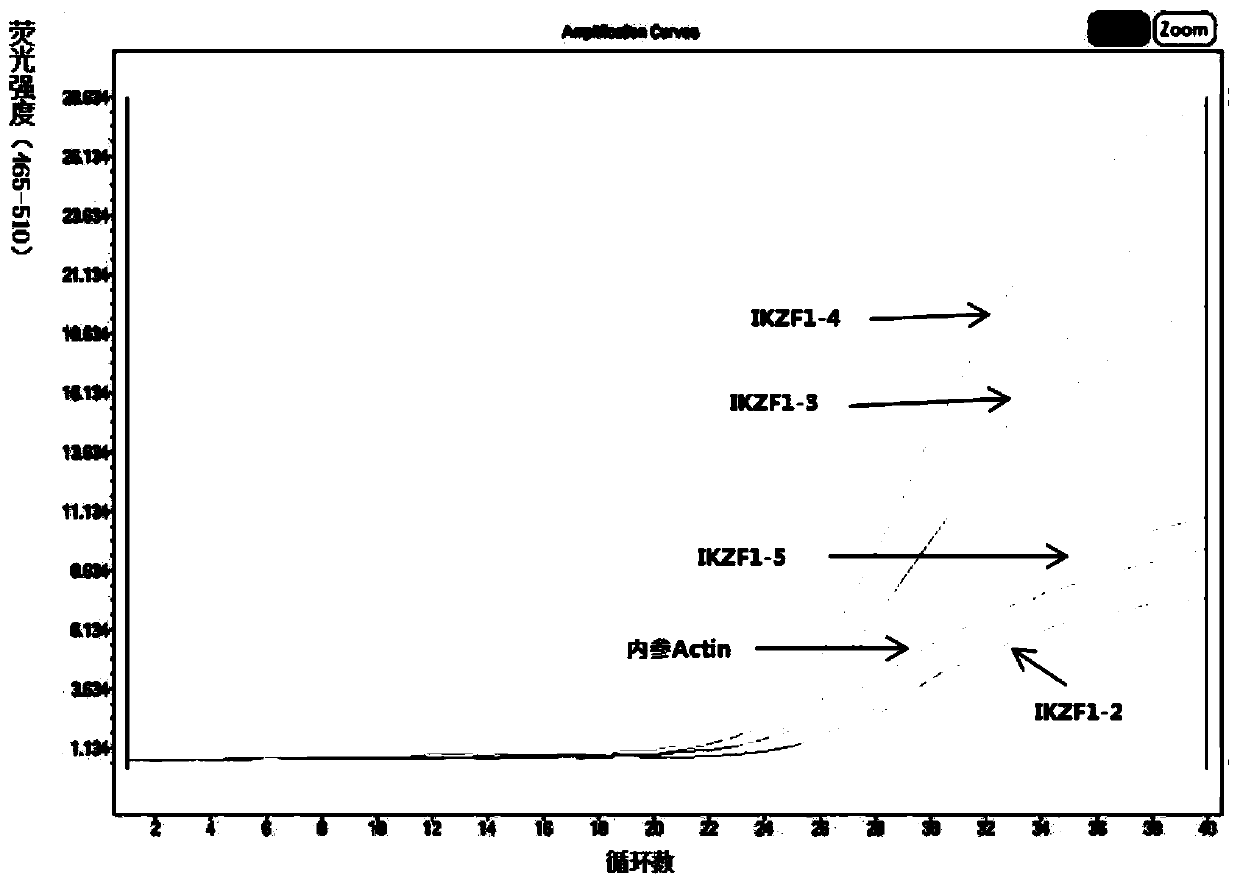
[0134] Prepare PAX5 gene (1-10) and IKZF1 gene (2-8) mutation detection kits, specifically comprising the following steps:
[0135] 1. Synthetic primers and probe sequences
[0136] Synthesize PCR primer probe sequences SEQ ID NO.1-SEQ ID NO.51, wherein the 5' end of the specific probe sequence is labeled with a FAM fluorescent group. Synthesize the internal standard primer probe sequence SEQ ID NO.52-SEQ ID NO.54, wherein, the internal standard probe sequence SEQ ID NO.54 is labeled with a FAM fluorescent group at the 5' end and a BHQ1 quencher at the 3' end group.
[0137] The above primer sequences (or internal standard primers) were respectively prepared into 100 pmol / ul stock solution for storage, and the above probe sequences (internal standard probes) were prepared respectively for storage in stock solution.
[0138] 2. Preparation of fluorescent quantitative PCR reaction system
[0139] Prepare mutation detection reaction systems containing No. 1-17 reagents respect...
Embodiment 2
[0144] Use the PAX5 and IKZF1 gene mutation detection kit prepared in Example 1 to detect the samples to be tested.
[0145] In this embodiment, 16 cases (numbering B1-B16)) of newly diagnosed, refractory and relapsed B-ALL patients (16 patients including children and adults) were collected and tested through informed consent (children were relatives). Agree to detect) blood samples and extract genomic DNA therefrom, detect whether there is corresponding gene mutation in the sample to be tested with the PAX5 and IKZF1 gene mutation detection kit that obtains in embodiment 1, concrete operation steps are:
[0146] 1. Extraction of Genomic DNA from Samples
[0147] Using a DNA extraction kit (The Blood DNA Kit (purchased from Beijing Quanshijin Biotechnology Co., Ltd.) was used to extract the genomic DNA of the blood samples of the above-mentioned B-ALL patients and the genomic DNA of normal persons. NANO300 was used to detect the concentration of DNA. The A260 / A280 value of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com