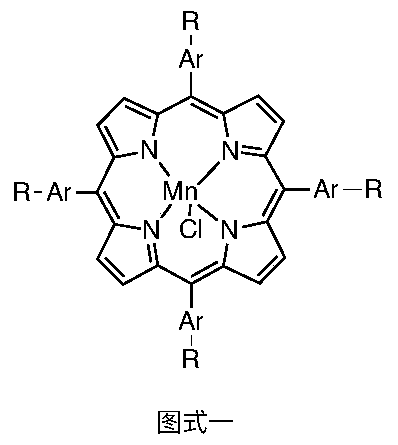
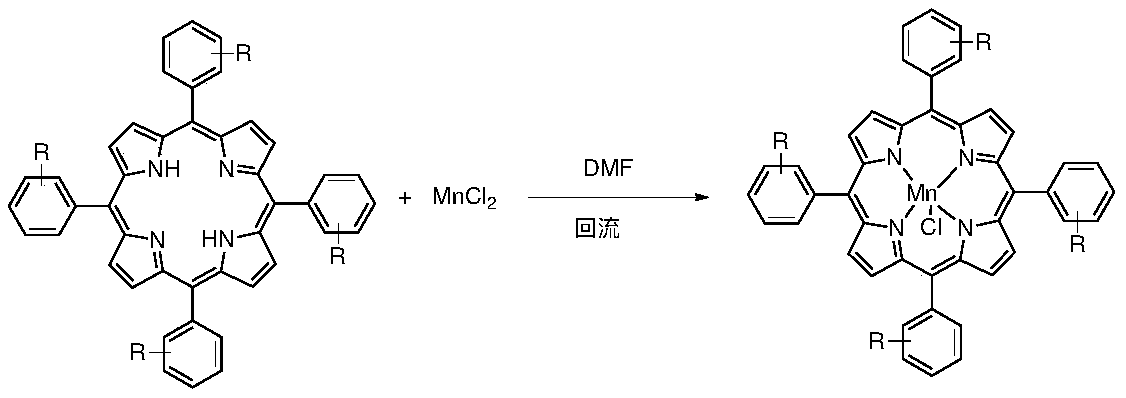
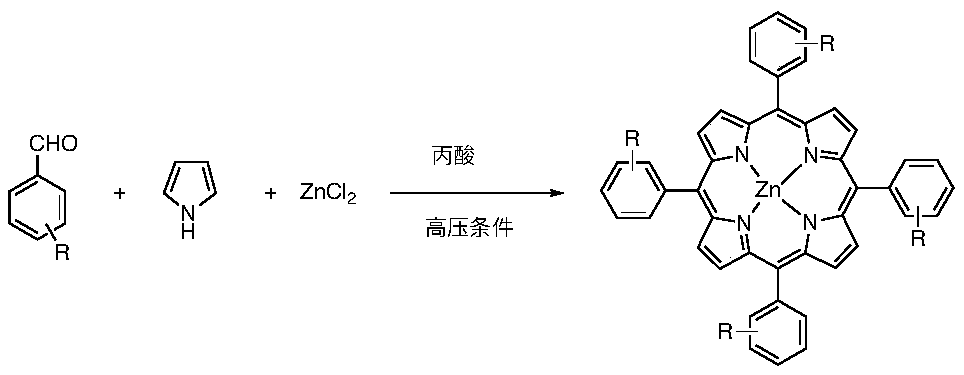
Method for synthesizing tetraaryl manganese porphyrin through synchronous aldehyde and pyrrole condensation and bivalent manganese salt oxidation insertion reaction
A technology of tetraaryl manganese porphyrin and divalent manganese salt is applied in the field of organic synthesis to achieve the effects of improving condensation yield, simplifying separation process and reducing reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 250mL DMF to a stirred reactor with a reflux condenser, add 0.75 moles of anhydrous aluminum trichloride, 0.5 moles of benzaldehyde, 0.5 moles of pyrrole and 1.5 moles of manganese dichloride successively under stirring, and heat the reaction mixture to reflux After the reaction was maintained for 2 hours, the reaction was stopped, the temperature was lowered, and the mixture was placed at 273K overnight, and the tetraphenylporphyrin manganese purple black crystals were obtained by suction filtration, with a yield of 50%.
Embodiment 2
[0032] Add 240mL DMF to a stirred reactor with a reflux condenser, add 1 mole of anhydrous aluminum trichloride, 0.8 mole of 3-bromopyridine formaldehyde, 0.6 mole of pyrrole and 1.5 mole of manganese dichloride in turn under stirring, and heat to react The mixture was refluxed and maintained for 1 hour, then the reaction was stopped, the temperature was lowered, and it was placed overnight at 273K, and filtered by suction to obtain purple-black crystals of tetrakis(3-bromopyridine)porphyrin manganese, with a yield of 35%.
Embodiment 3
[0034] Add 210mL DMF into a stirred reactor with a reflux condenser, add 1.2 moles of anhydrous aluminum trichloride, 0.8 moles of 2-ethylpyrrole formaldehyde, 0.7 moles of pyrrole and 2 moles of manganese dichloride in turn under stirring, and heat The reaction mixture was refluxed and maintained for 2 hours, then the reaction was stopped, the temperature was lowered, and it was placed at 273K overnight, and filtered by suction to obtain tetrakis(2-ethylpyrrole) porphyrin manganese purple black crystals, with a yield of 30%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com