Detection kit for simultaneously detecting nine respiratory tract viruses including SARS-CoV-2
A detection kit, sars-cov-2 technology, applied in the direction of microorganism-based methods, microorganism measurement/testing, microorganisms, etc., can solve the problems of large demand and achieve high sensitivity, good specificity, and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
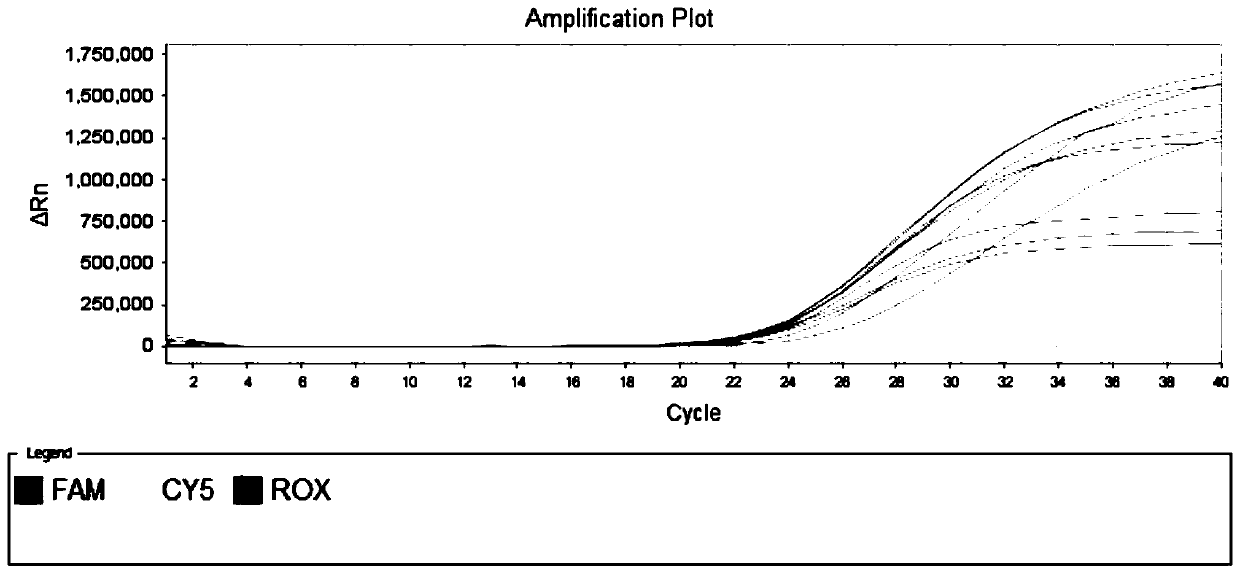
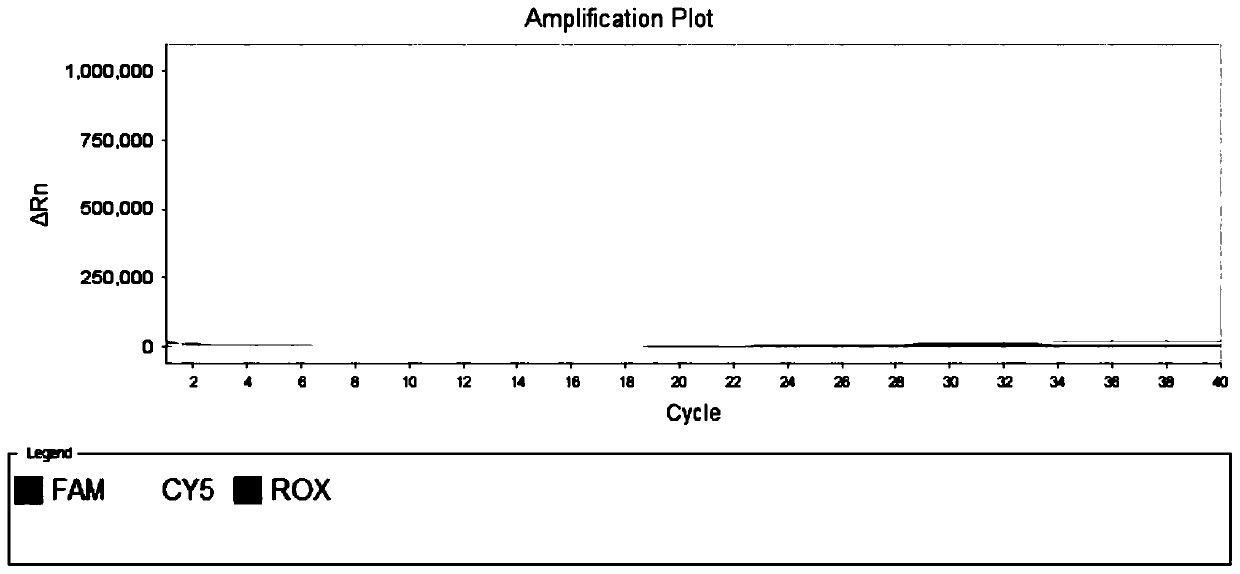
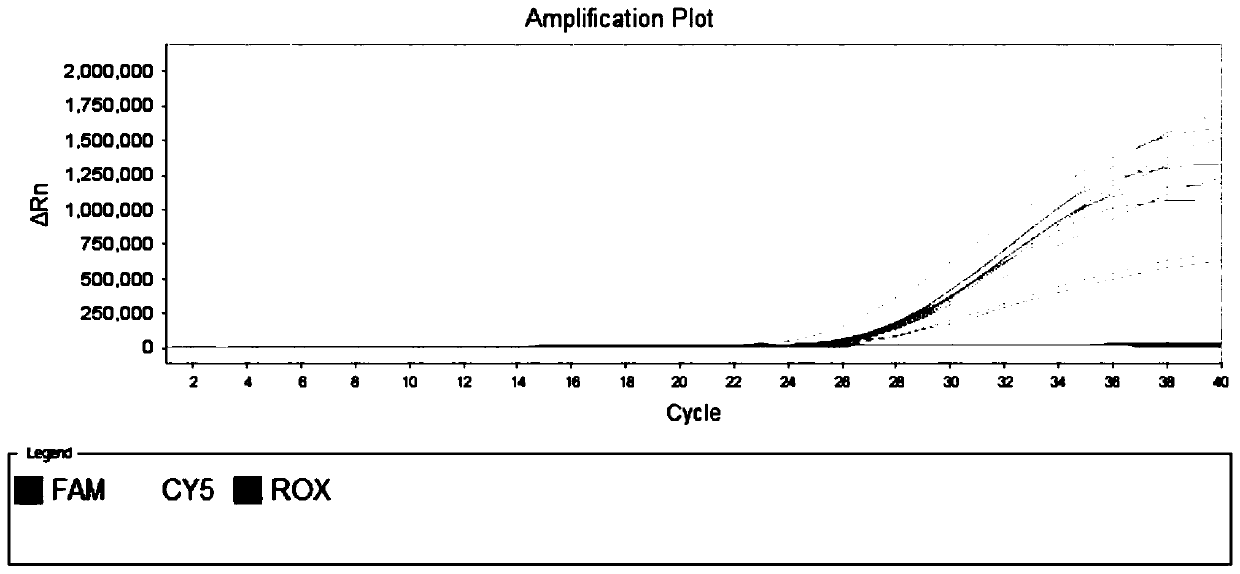
[0061] Example 1 is used to specifically detect the primer set and fluorescent probe of novel coronavirus SARS-CoV-2
[0062] Download the sequence of the new coronavirus SARS-CoV-2 published in the GISAID database, use the MAFFT analysis software to compare the sequences, and analyze the conserved regions of its genome. Dozens of specific primers and probe sequences for the detection of the novel coronavirus ORF1ab gene were designed in these conserved regions.
[0063] The design of primers and probes follows the following principles:
[0064] Choose the probe first, and then design the primers to be as close to the probe as possible. The selected sequence should be highly specific, and try to choose an amplified fragment with minimal secondary structure; this is because secondary structure will affect the efficiency of the reaction, and it will also hinder the amplification of the enzyme. The secondary structure detection is carried out first. If the secondary structure c...
Embodiment 2
[0090] Embodiment 2 simultaneously detects the detection kit of nine kinds of respiratory viruses that comprise SARS-CoV-2
[0091] A detection kit for simultaneous detection of nine respiratory viruses including SARS-CoV-2, including detection of influenza A virus, influenza B virus, new coronavirus SARS-CoV-2, rhinovirus, parainfluenza virus, respiratory syndrome Primer sets and probe sets for cellular virus, coronavirus 229E subtype, coronavirus OC43 subtype, coronavirus HKU1 subtype; and reaction solutions, enzyme mixtures, positive quality controls, and negative quality controls.
[0092] specifically,
[0093] The sequence of the upstream primer for detecting influenza A virus is shown in SEQ ID No.1, the sequence of the downstream primer is shown in SEQ ID No.2, and the sequence of the fluorescent probe is shown in SEQ ID No.3;
[0094] The sequence of the upstream primer for detecting influenza B virus is shown in SEQ ID No.4, the sequence of the downstream primer is ...
Embodiment 3
[0123] Embodiment 3 adopts the method for the kit of embodiment 2 to detect nine kinds of respiratory viruses simultaneously
[0124] 1. To extract the nucleic acid of the sample to be tested, use the nucleic acid extraction kit (magnetic bead method) of Shandong Aikewei Biotechnology Co., Ltd. (Luji Machinery No. 20190256).
[0125] 2. Prepare the reaction system, prepare the reaction solution, primer-probe mixture, and enzyme mixture in the kit according to the kit instructions.
[0126] Table 3 shows the preparation methods for detecting influenza A virus, influenza B virus, and novel coronavirus system.
[0127] Table 3 Detection of influenza A virus, influenza B virus, novel coronavirus SARS-CoV-2 system preparation method
[0128] Reaction system components Additional amount (μL) / per person Enzyme Mix 4 The reaction solution 12 Primer Probe Mix A 4 total capacity 20
[0129] The preparation method of the detection system for rhin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com