Method for preparing high-carbon aldehyde
A high-carbon aldehyde and high-carbon olefin technology, applied in the field of high-carbon aldehyde preparation, can solve the problems of catalytic reaction rate that cannot meet industrial production, low mass transfer rate between two phases, and poor solubility of high-carbon olefins. Good performance, reduced manufacturing cost and maintenance cost, and high quality ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
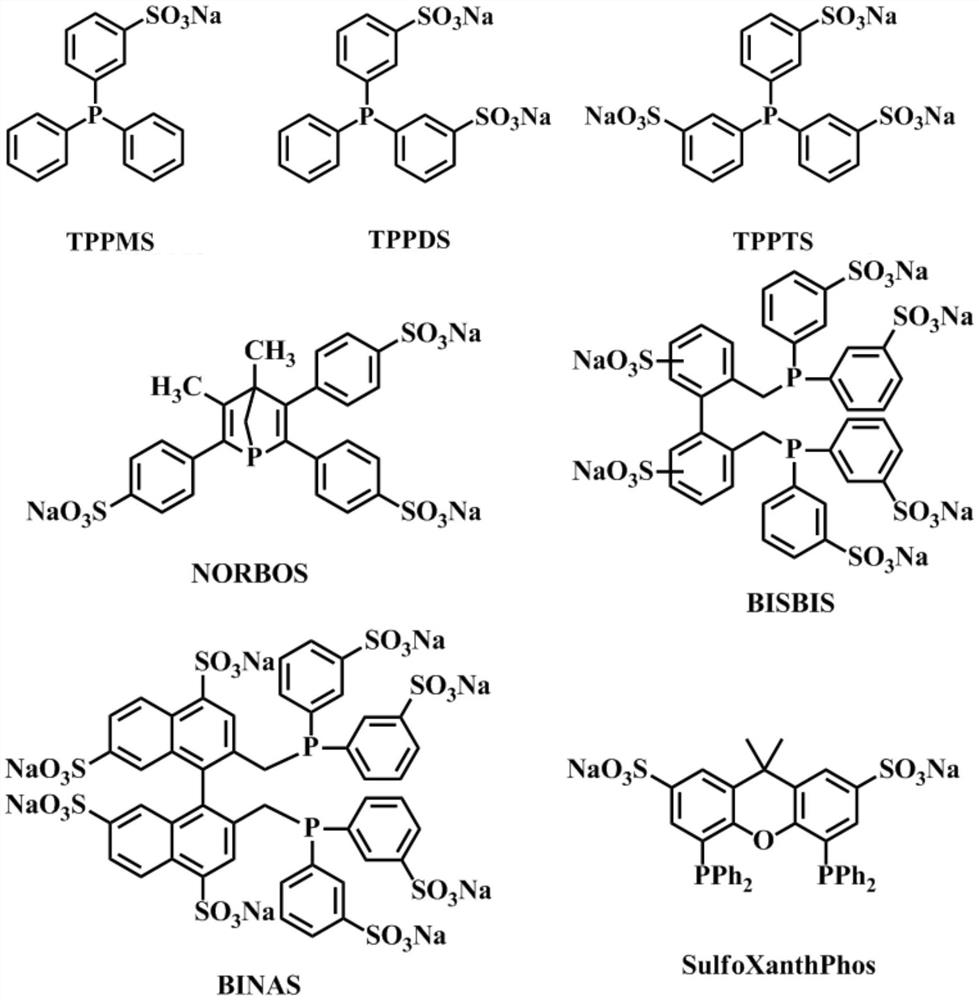
[0046] With 0.115 gram of rhodium catalyst HRh (CO) (TPPTS) 3 , 0.68 gram of BISBIS, 10 gram of diethylene glycol and 20 gram of methyl alcohol, 10 grams of 1-dodecene, respectively add in the autoclave with stirrer and temperature controller, feed hydrogen and carbon monoxide mol ratio in the still and be 1.0:1.0 Replace the air in the kettle for 3 to 5 times, then continue to feed the synthesis gas into the kettle until the total pressure in the kettle reaches 3.0MPa, react at a temperature of 120°C and a stirring speed of 1200rpm for 1.0 hour, cool to room temperature, and release After the remaining gas, the reaction liquid was analyzed by gas chromatography, and the conversion rate of 1-dodecene was calculated to be 98.0%, the selectivity of tridecanal to be formed was 95.1%, and the selectivity of linear aldehyde was 95.3%.
Embodiment 2
[0048]With 0.115 gram of rhodium catalyst HRh (CO) (TPPTS) 3 , 2 grams of BISBIS, 10 grams of water and 30 grams of methanol, 10 grams of 1-dodecene, add respectively in the autoclave with stirrer and temperature controller, feed hydrogen and carbon monoxide in the still with a molar ratio of 1.0:1.0 Synthesis gas, replace the air in the kettle 3 to 5 times, then continue to feed synthesis gas into the kettle until the total pressure in the kettle reaches 3.0MPa, keep the pressure constant, react at a temperature of 120°C and a stirring speed of 1200rpm for 1.5 hours, and cool to room temperature , after releasing the remaining gas, the reaction solution was analyzed by gas chromatography, and the conversion rate of 1-dodecene was calculated to be 99.5%, and the selectivity of tridecaldehyde to generate was 96.8%, and the selectivity of straight-chain aldehyde was 98.3%. %.
Embodiment 3
[0050] With 0.115 gram of rhodium catalyst HRh (CO) (TPPTS) 3 , 0.4 gram of BISBIS, 10 gram of triethylene glycol and 30 gram of methyl alcohol, 10 gram of 1-octene, respectively add in the autoclave of belt stirrer and temperature controller, feed hydrogen and carbon monoxide molar ratio in the still and be 1.05:1.0 Synthesis gas, replace the air in the kettle for 3 to 5 times, then continue to feed synthesis gas into the kettle until the total pressure in the kettle reaches 2.5MPa, keep the pressure constant, react at a temperature of 115°C and a stirring speed of 1200rpm for 1.5 hours, and cool to room temperature After releasing the remaining gas, the reaction solution was analyzed by gas chromatography, and the conversion rate of 1-octene was calculated to be 98.8%, the selectivity of nonanal to be generated was 96.3%, and the selectivity of linear aldehyde was 97.0%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com