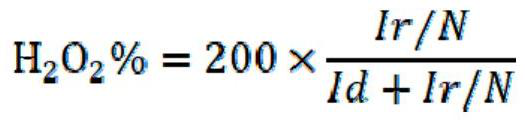Nano-catalyst for electrochemical preparation of hydrogen peroxide, and preparation method and application thereof
A nano-catalyst, hydrogen peroxide technology, applied in physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, catalyst activation/preparation, etc., can solve high overpotential, limited catalytic activity, adsorption capacity Poor problems, to achieve the effect of increased selectivity and activity, high-quality activity, and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
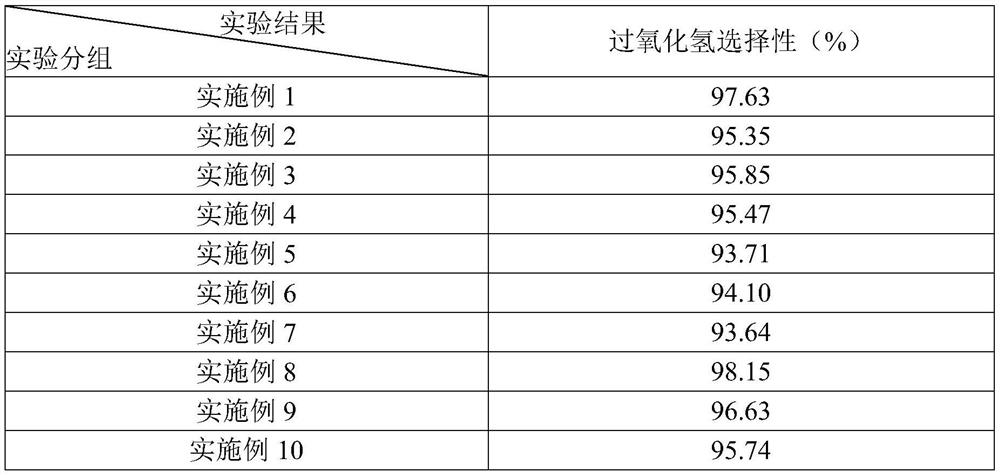
Examples
Embodiment 1
[0036]This embodiment provides an electrocatalytic material for producing hydrogen peroxide, which is prepared through the following steps:
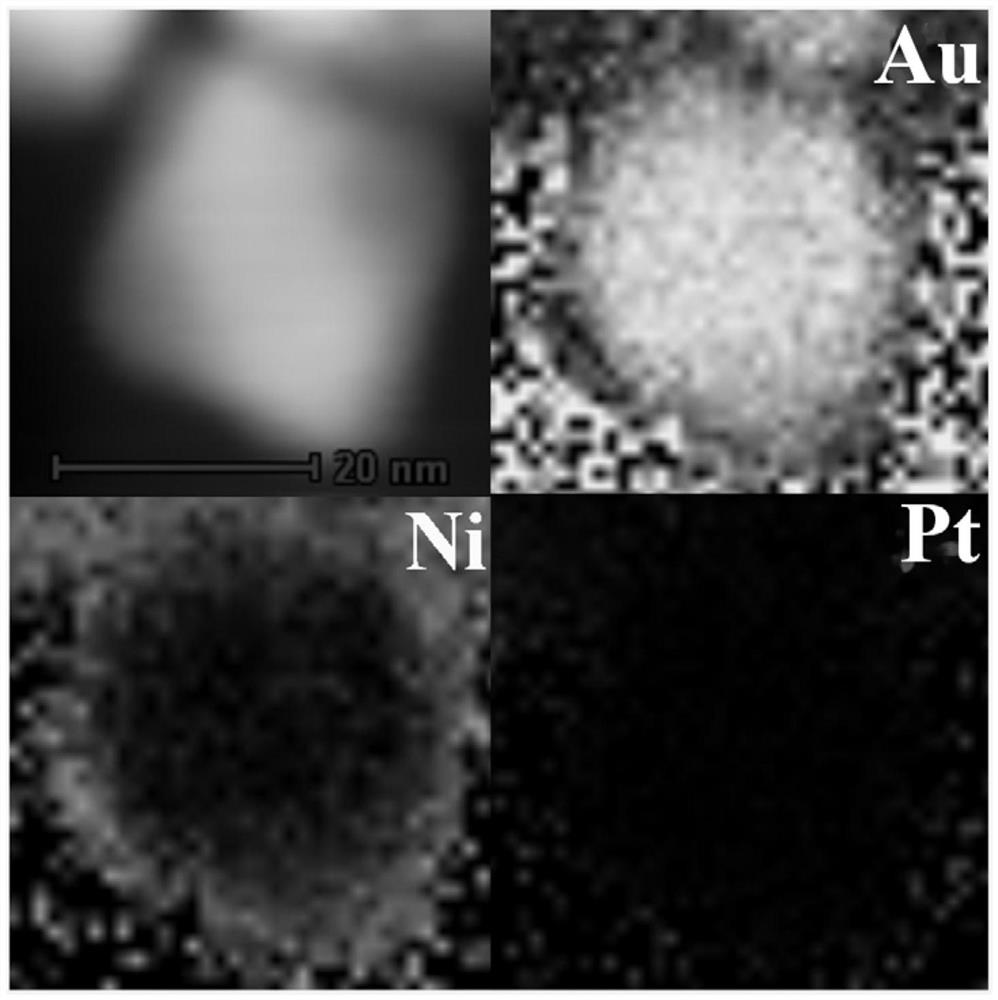
[0037] Add 0.6mL of freshly prepared sodium borohydride (0.01M) ice-water solution to a mixed solution of 0.1M chloroauric acid (0.025mL) and cetyltrimethylammonium bromide (10mL) to prepare into a seed solution. The above mixed solution was vigorously stirred for 2 minutes before use, and aged at room temperature for about 5 minutes before use.
[0038] Dissolve 7.288g of cetyltrimethylammonium bromide in 100mL of warm water at 55°C to make a solution with a concentration of 0.2M, add 2mL of silver nitrate solution with a concentration of 0.01M to the solution, and let it stand at room temperature for 10 Minutes later, add 100mL of chloroauric acid solution with a concentration of 0.8mM, stir the mixed solution gently for about 10 minutes, add 0.9mL of freshly prepared ascorbic acid solution (0.1M), stir rapidly until the solution beco...
Embodiment 2
[0044] This embodiment provides an electrocatalytic material for producing hydrogen peroxide, which is prepared through the following steps:
[0045] Add 0.6mL of freshly prepared sodium borohydride (0.01M) ice-water solution to a mixed solution of 0.1M chloroauric acid (0.025mL) and cetyltrimethylammonium bromide (10mL), Make a seed solution. The above mixed solution was vigorously stirred for 2 minutes before use, and aged at room temperature for about 5 minutes before use.
[0046] Dissolve 7.288g of cetyltrimethylammonium bromide in 100mL of warm water at 55°C to make a solution with a concentration of 0.2M, add 2mL of silver nitrate solution with a concentration of 0.01M to the solution, and let it stand at room temperature for 10 Minutes later, add 100mL of chloroauric acid solution with a concentration of 0.8mM, stir the mixed solution gently for about 10 minutes, add 0.9mL of freshly prepared ascorbic acid solution (0.1M), stir rapidly until the solution becomes color...
Embodiment 3
[0051] This embodiment provides an electrocatalytic material for producing hydrogen peroxide, which is prepared through the following steps:
[0052] Add 0.6mL of freshly prepared sodium borohydride (0.01M) ice-water solution to a mixed solution of 0.1M chloroauric acid (0.025mL) and cetyltrimethylammonium bromide (10mL) to prepare into a seed solution. The above mixed solution was vigorously stirred for 2 minutes before use, and aged at room temperature for about 5 minutes before use.
[0053] Dissolve 7.288g of cetyltrimethylammonium bromide in 100mL of warm water at 55°C to make a solution with a concentration of 0.2M, add 2mL of silver nitrate solution with a concentration of 0.01M to the solution, and let it stand at room temperature for 10 minutes Finally, add 100mL of chloroauric acid solution with a concentration of 0.8mM, stir the mixed solution gently for about 10 minutes, add 0.9mL of freshly prepared ascorbic acid solution (0.1M), and stir rapidly until the soluti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com