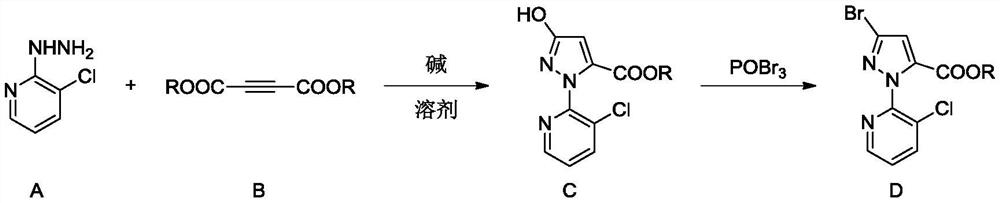
A kind of preparation method of 1-(3-chloropyridin-2-yl)-3-bromo-1h-pyrazole-5-carboxylate
A technology of chloropyridine and formate, which is applied in the field of preparation of 1--3-bromo-1H-pyrazole-5-carboxylate, can solve the problems of being unsuitable for industrial production, poor atom economy, long steps, etc. Achieve the effects of avoiding the use of oxidizing reagents, less waste, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] In a 250ml round bottom flask, add 20g (0.139mol) of 3-chloro-2-hydrazinopyridine, 100g of methanol, 9g (0.166mol) of sodium methylate, heat up to reflux, and dropwise add 24.7g of raw material B (R=Me) ( 0.174mol), dripped for 1 hour, refluxed for 2 hours, cooled to 50°C, added 12g of glacial acetic acid, adjusted the pH to 6-7, recovered methanol under negative pressure, then added 100g of water and 20g of methanol, slowly cooled to 0°C, pumped After filtering and drying, 26.5 g of product C (R=Me) was obtained, with a content of 98.7% and a yield of 74.0%.
Embodiment 2
[0036] Add 40g (0.279mol) of 3-chloro-2-hydrazinopyridine, 80g ethanol, and 22.7g (0.333mol) of sodium ethoxide into a 250ml round bottom flask, heat up to reflux, and add 59.2g of raw material B (R=Et) dropwise (0.348mol), after 1 hour of dripping, reflux for 1 hour, cool down to 50°C, add 26g of glacial acetic acid, adjust the pH to 6-7, recover ethanol under negative pressure, then add 150g of water and 40g of ethanol, slowly cool down to 0°C, After suction filtration and drying, 59.8 g of product C (R=Et) was obtained, with a content of 98.5% and a yield of 78.8%.
Embodiment 3
[0038] In a 250ml round bottom flask, add 40g (0.279mol) of 3-chloro-2-hydrazinopyridine, 80g of tert-butanol, 33.1g (0.345mol) of sodium tert-butoxide, heat up to reflux, and dropwise add raw material B (R = Et) 60.4g (0.355mol), 1h dripping, reflux for 1h, cool down to 50°C, add 26g of glacial acetic acid, adjust the pH to 6-7, recover the solvent under negative pressure, then add 180g of water and 20g of ethanol, slowly cool down to 0°C, suction filtered, and dried to obtain 49.2 g of product C (R=Et), with a content of 97.2% and a yield of 64%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com