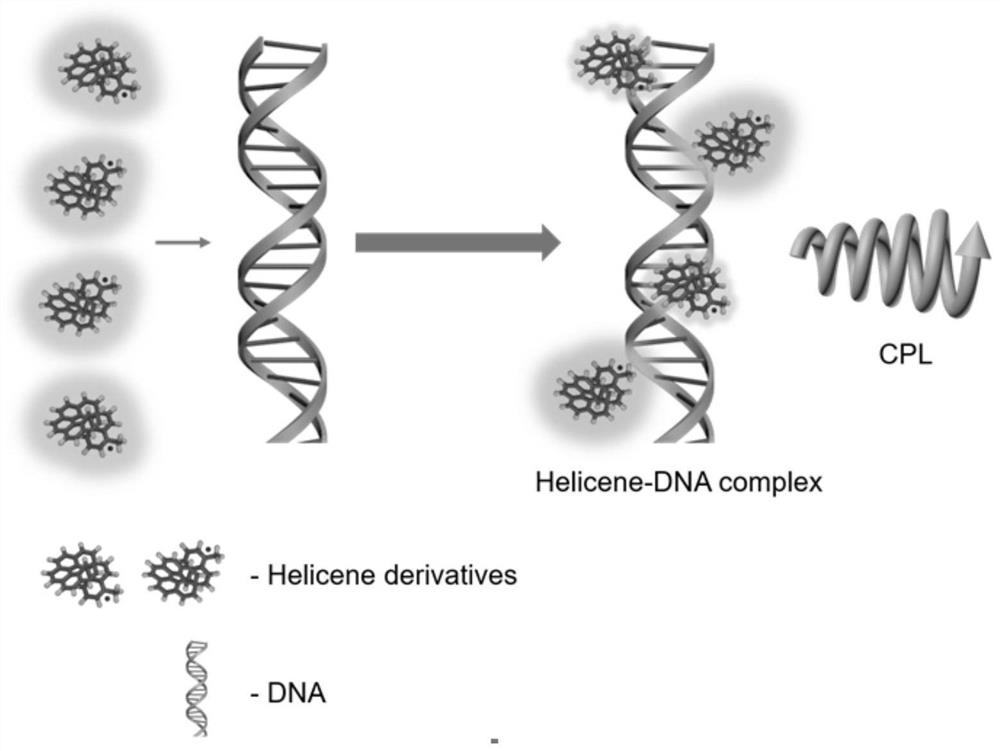
Axial chiral azahelicene derivative, circular polarization luminescent material and preparation method
A technology of azahelicene and luminescent materials, applied in luminescent materials, organic chemical methods, chemical instruments and methods, etc., can solve the problem of low asymmetry factor, reduce difficulty, excellent chiral luminescence performance, and expand the selection range Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] This embodiment relates to the method for preparing racemic azahelicene A, as follows:
[0044]
[0045] The reaction principle is as figure 1 As shown, it specifically includes the following steps: 2.4g of 2-methyl[4]helicene bromotriphenylphosphine salt is dissolved in 20mL of tetrahydrofuran dehydrated, and cooled to -78°C with an ice ethanol bath under nitrogen protection. Slowly add 2.8 mL of n-butyllithium (1.6 mol / L n-hexane solution) to the reaction system, react at -78°C for 5 minutes, then slowly raise the temperature to room temperature for 30 minutes. As the reaction progressed, the reaction system gradually turned dark red. Then the reaction system was cooled to -78°C with an ice ethanol bath, and 628 mg of 6-formylquinoline was added thereto, reacted at -78°C for 5 minutes, and then slowly warmed up to room temperature for 60 minutes. After the reaction was completed, the solvent was removed and then purified with a silica gel column (eluent: ethyl ac...
Embodiment 2
[0049] This embodiment relates to the method for preparing chiral azahelicene, the process is as follows:
[0050]
[0051] Specifically, chiral resolution is performed using a high-performance liquid chromatography chiral column, and the specific chiral column is a CHIRALPAKIG preparative column; the mobile phase is n-hexane / ethanol=80 / 20 (v / v); the P-type isomer is obtained by resolution I (e.e. >99%) and M-type isomer II (e.e. >99%).
[0052] The high-performance liquid chromatography data of this compound are shown in table 1 and table 2, and relevant spectrogram sees Figure 4 with Figure 5 shown;
[0053] Table 1, the high performance liquid chromatography data of compound I
[0054] compound Ret. Time area Area% T.Plate# I 4.949 3111176 99.9797 654.005 II 5.812 630 0.0203 12499.444
[0055] Table 2, HPLC data of compound II
[0056] compound Ret. Time area Area% T.Plate# I 4.951 1629 0.0399 5651.0...
Embodiment 3
[0060] This embodiment relates to a preparation method of azahelicene quaternary ammonium salt, such as Figure 8 As shown, it specifically includes the following steps: Preparation of Compound B: Weigh 38 mg of Compound A, add about 0.2 mL of methyl iodide, and react for 24 hours under stirring at room temperature. After the reaction was completed, 50 mg of an orange solid was obtained after removal of iodomethane, with a yield of 96%.
[0061] Chiral compounds III and IV can be obtained by the same method using compounds I and II as reactants, and the yields are 98% and 94%, respectively.
[0062] The characterization results of compound B are as follows:
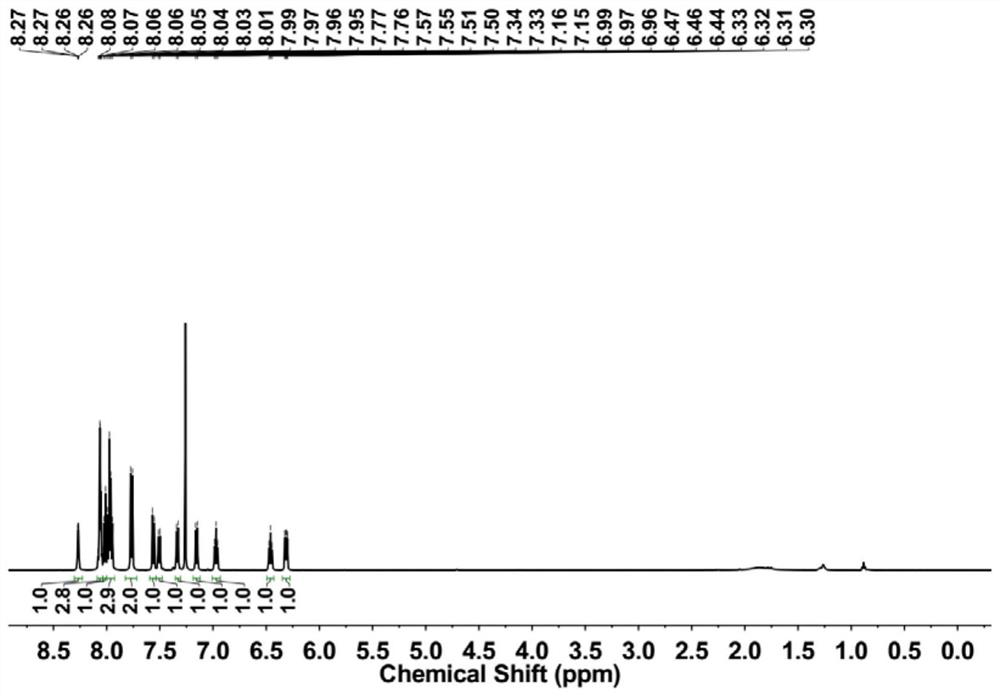
[0063] 1 H NMR (500MHz, DMSO-d6, 295K): δ8.78(d, J=5.6Hz, 1H), 8.73(d, J=9.2Hz, 1H), 8.48(d, J=8.2Hz, 1H), 8.40(d,J=8.2Hz,1H),8.34(d,J=8.3Hz,2H),8.31-8.24(m,2H),8.21(d,J=9.2Hz,2H),7.99(d,J =8.5Hz, 1H), 7.72(d, J=8.5Hz, 1H), 7.51(d, J=7.9Hz, 1H), 7.12(t, J=7.4Hz, 1H), 7.03-6.92(m, 2H ), 6.49(t, J=7.6Hz, 1H), 4.36(s, 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com