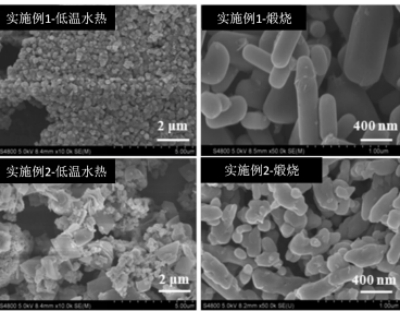
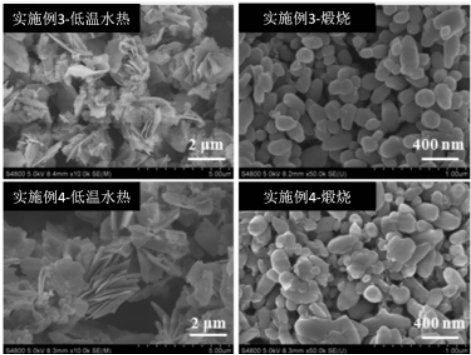
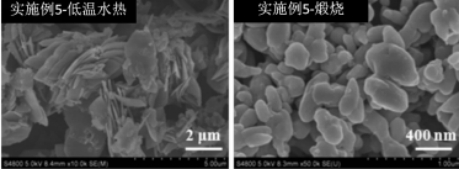
Preparation method and application of lithium iron manganese phosphate cathode material
A technology of lithium iron manganese phosphate and positive electrode materials, applied in the direction of phosphate, phosphorus oxyacids, chemical instruments and methods, etc., can solve the problems of poor cycle stability, low reversible capacity, large electronic transition energy gap, etc., and achieve energy saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] A preparation method of lithium iron manganese phosphate positive electrode material, the preparation method is as follows:
[0027] S1, the reaction raw material by product LiFe 0.3 mn 0.7 PO 4 The stoichiometric ratio is accurately weighed;
[0028] S2, putting the reaction raw materials in step S1 into a high-energy ball mill to perform high-energy ball mill pre-reaction;
[0029] S3. After the high-energy ball milling is completed, the product in step S3 is separated, and transferred to deionized water for dispersion, and then low-temperature hydrothermal reaction is carried out;
[0030] S4. After the low-temperature hydrothermal reaction is completed, the product in step S4 is separated, ground in an agate mortar, and then calcined.
[0031] Specifically, the lithium source used in the present invention is lithium chloride, the phosphorus source used is ammonium dihydrogen phosphate, the manganese source used is manganese dioxide, and the iron source used is f...
Embodiment 1
[0036] By product LiFe 0.3 mn 0.7 PO 4The stoichiometric ratio of reaction materials Lithium Chloride, Ammonium Dihydrogen Phosphate, Manganese Dioxide and Ferrous Chloride were weighed, the above materials were put into a high-energy ball mill, and the high-energy ball mill was high-energy ball milled at a speed of 3200rpm for 40min; Take it out, add deionized water, react in a hydrothermal reactor at 80°C for 1.5h, take it out and cool it down to room temperature naturally, then separate it by centrifugal filtration, and then dry it in a vacuum oven at 100°C, the dried product Grinding in an agate mortar, and then calcining in a muffle furnace at 500°C for 7 hours under the protection of argon to obtain the final product LiFe 0.3 mn 0.7 PO 4 Cathode material.
Embodiment 2
[0038] By product LiFe 0.3 mn 0.7 PO 4 The stoichiometric ratio of the reaction materials was taken by weighing lithium chloride, ammonium dihydrogen phosphate, manganese dioxide and ferrous chloride, and the above-mentioned substances were put into a high-energy ball mill, and high-energy ball milled at a speed of 3500rpm for 30min; Take it out, add deionized water, react in a hydrothermal reactor at 80°C for 2 hours, take it out and cool it to room temperature naturally, then separate it by centrifugal filtration, and then dry it in a vacuum oven at 100°C. The dried product is in Grinding in an agate mortar, and then calcining in a muffle furnace at 500°C for 7 hours under the protection of argon to obtain the final product LiFe 0.3 mn 0.7 PO 4 Cathode material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com