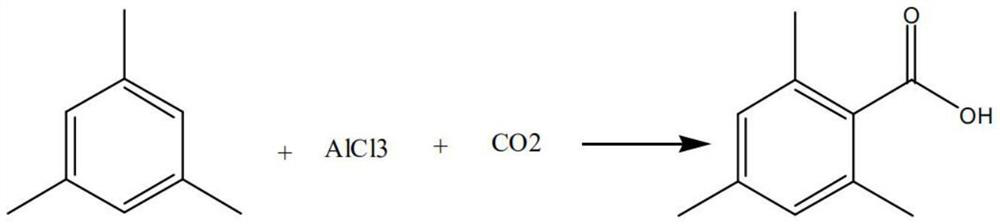
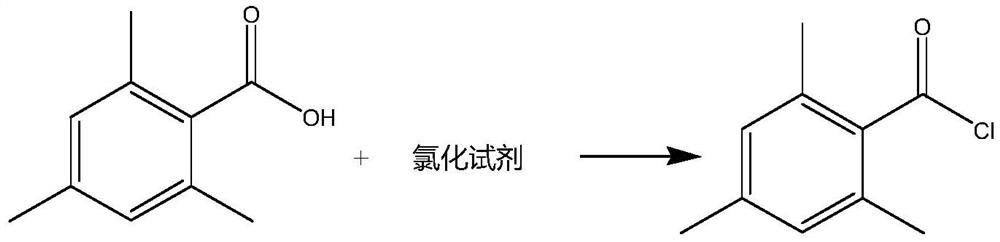
Preparation method of 2, 4, 6-trimethylbenzoyl chloride
A technology for trimethylbenzoyl chloride and crude trimethylbenzoyl chloride is applied in the field of chemical industry, can solve problems such as low reaction yield, sharp reaction, high cost organic waste liquid, etc. The effect of simplifying operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Friedel-Crafts reaction: add 368g aluminum chloride and 662g mesitylene in the autoclave, stir, then pass into carbon dioxide and react at 25°C under 0.35MPa;
[0030] (2) Hydrolysis and post-treatment: Add the reaction solution after the reaction in step (1) dropwise to the hydrolyzate, wherein the hydrolyzate is a mixed solution of 150g hydrochloric acid and 900g water, and carry out at a temperature not higher than 80°C Hydrolysis reaction, static separation after hydrolysis; take the organic layer and add 900g of water to the organic layer to obtain a distilled mixed solution, heat up to 98°C and azeotropically reflux to distill out the mesitylene, wherein the distilled mesitylene is passed After freezing and dehydration, circulate it as the solvent for the next batch of Friedel-Crafts reaction. During the reflux process, stir the distillation mixture at a speed of 650r / min. When no mesitylene is distilled out, keep the temperature at 100°C for 45 minutes, then c...
Embodiment 2
[0033] (1) Friedel-Crafts reaction: add 368g aluminum chloride and 405g mesitylene in the autoclave, stir, then pass into carbon dioxide to react at 30 ℃ under 0.3MPa;
[0034] (2) Hydrolysis and post-treatment: Add the reaction solution after the reaction in step (1) dropwise to the hydrolyzate, wherein the hydrolyzate is a mixed solution of 150g hydrochloric acid and 900g water, and carry out at a temperature not higher than 80°C Hydrolysis reaction, static separation after hydrolysis; take the organic layer and add 900g of water to the organic layer to obtain a distilled mixed solution, heat up to 95°C and azeotropically reflux to evaporate mesitylene, wherein, the steamed mesitylene is passed After freezing and dehydration, it can be used as the next batch of Friedel-Crafts reaction solvent. During the reflux process, the distillation mixture is stirred at a speed of 500r / min. When no mesitylene is distilled out, keep the temperature at 100°C for 30 minutes, then cool to ro...
Embodiment 3
[0037] (1) Friedel-Crafts reaction: add 368g aluminum chloride and 1100g mesitylene to the autoclave, stir, then feed carbon dioxide to react at 20°C under 0.4MPa;
[0038] (2) Hydrolysis and post-treatment: Add the reaction solution after the reaction in step (1) dropwise to the hydrolyzate, wherein the hydrolyzate is a mixed solution of 150g hydrochloric acid and 900g water, and carry out at a temperature not higher than 80°C Hydrolysis reaction, static separation after hydrolysis; take the organic layer and add 900g of water to the organic layer to obtain a distilled mixed solution, heat up to 98°C and azeotropically reflux to distill out the mesitylene, wherein the distilled mesitylene is passed After freezing and dehydration, it can be used as the next batch of Friedel-Crafts reaction solvent. During the reflux process, the distillation mixture is stirred at a speed of 800r / min. When no mesitylene is distilled out, keep the temperature at 100°C for 60 minutes, then cool to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com