Antibodies binding ctla-4 and uses thereof
An antibody and monoclonal antibody technology, applied in the direction of antibodies, antibody medical components, medical preparations containing active ingredients, etc., can solve the problem of low quantity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0151] Example 1 - Generation of Anti-CTLA-4 Antibodies
[0152] Human CTLA-4-ECD protein (Acro Bio) was used as an immunogen to generate anti-CTLA-4 antibodies. The use of human immunoglobulin transgenic mouse technology to develop and prepare human antibodies was first described in Abgenix (xeno mouse and Medarex (HuMab "mouse"); Lonberg et al., 1994, Nature, 368:856-859; Lonberg and Huszar, 1995, Internal Rev. Immunol., 13:65-93; Harding and Lonberg, 1995, Ann. N.Y. Acad. Sci., 764:536-546).
[0153]HCAb mice were immunized three times every two weeks with human CTLA-4-ECD protein at 20 mg / mouse, and six of them were immunized an additional five times at 44 mg / mouse. With the exception of the first injection, in which Stimune (Prionics) was used as adjuvant, all booster immunizations were performed with Ribi adjuvant (Sigma Adjuvant System S6322-1VL). After immunization, single cell suspensions were isolated from mouse bone marrow, spleen and lymph nodes. Mouse plasma ce...
Embodiment 2
[0165] Example 2-Preparation and purification of anti-CTLA-4 antibody
[0166] Step 1. Preparation of HEK 293F cells overexpressing hCTLA-4
[0167] The nucleotide sequence encoding human CTLA-4 (SEQ ID NO: 196, amino acid sequence encoding SEQ ID NO: 197) was subcloned into pcDNA3.1 vector (Clontech) to obtain a plasmid. HEK293 and CHO-K1 cells (Invitrogen) were transiently transfected with the plasmid using PEI, and the transformants were incubated in DMEM medium containing 0.5 g / mL penicillin / streptomycin and 10% (w / w) fetal bovine serum (FBS). cultured for 2 weeks. Limiting dilutions were performed in 96-well culture plates, and then incubated at 37°C with 5% (v / v) CO 2 Plates were incubated for approximately 2 weeks. Single clones were expanded in 6-well plates, and the expanded clones were screened by flow cytometry using a commercially available anti-hCTLA-4 antibody (R&D Systems). Clones showing higher growth rates and higher fluorescence intensities as measured by...
Embodiment 3
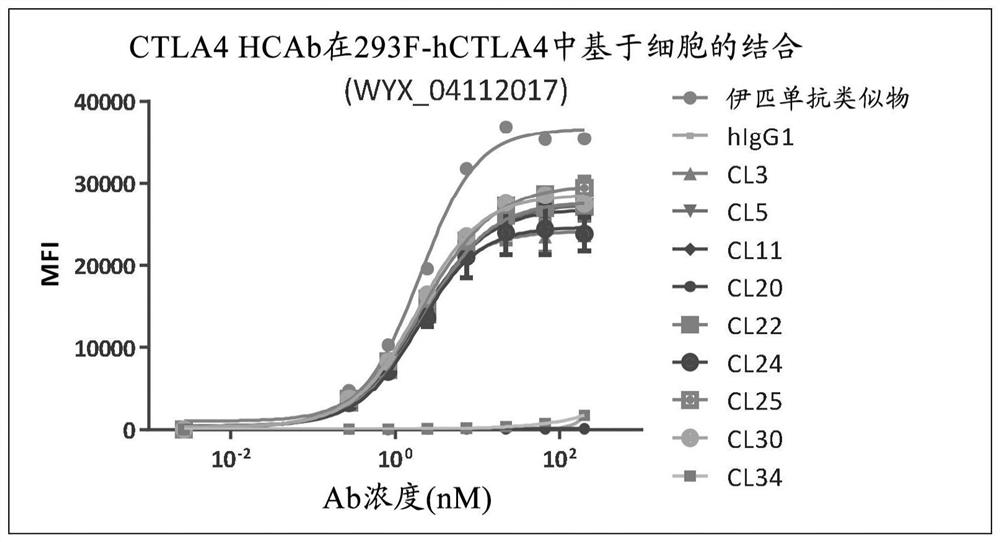
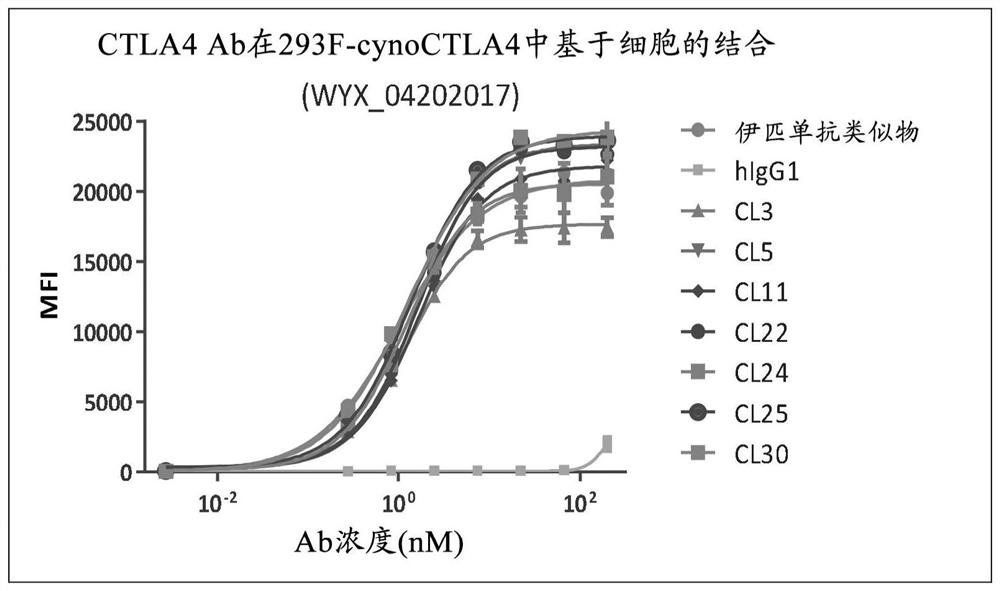
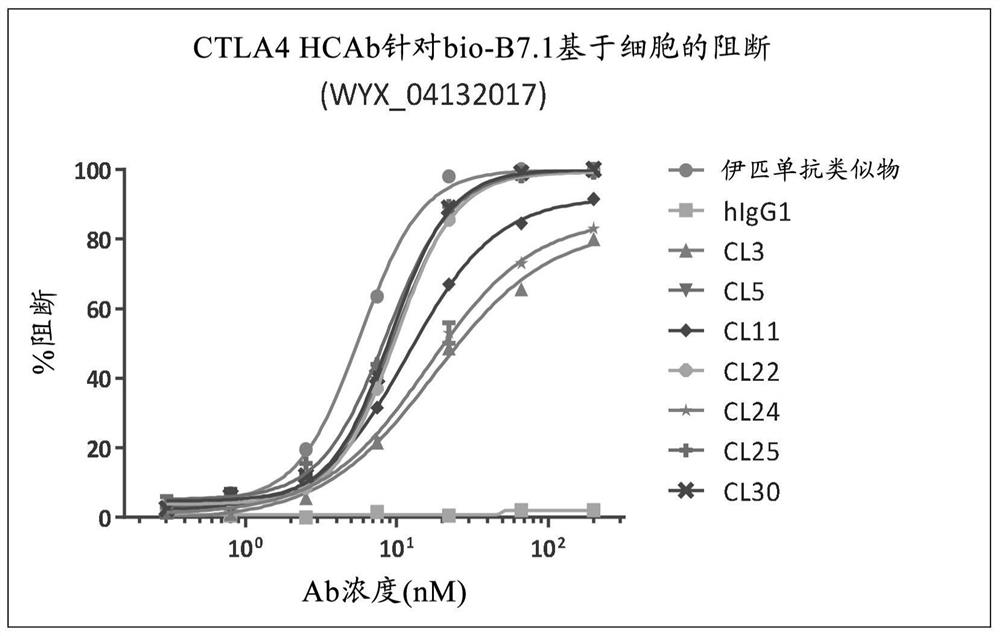
[0175] Example 3 - Characterization of lead candidate antibodies
[0176] 293 cells were stably transfected with the pTT5 plasmid containing the nucleic acid sequence encoding human CTLA-4 (SEQ ID NO: 196) to generate 293F cells stably expressing human CTLA-4 (herein referred to as 293-hCTLA-4 cells). Additional 293 cells were stably transfected with a pIRES plasmid containing the nucleic acid sequence encoding full-length cynomolgus CTLA-4 (SEQ ID NO: 198) to generate 293 cells stably expressing cynomolgus CTLA-4 (referred to herein as 293 -cynoCTLA-4 cells). 293-hCTLA-4 and 293-cynoCTLA-4 cells were cultured and expanded to 90% confluency in T-75 flasks. The medium was aspirated and the cells were washed twice with HBSS (Hanks' Balanced Salt Solution, Invitrogen). Cells were treated with enzyme-free cell dissociation solution (Versene solution, Invitrogen) and collected. Cells were then washed twice with HBSS, cell count determined, and 2x10 6 cells / mL and resuspend the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com