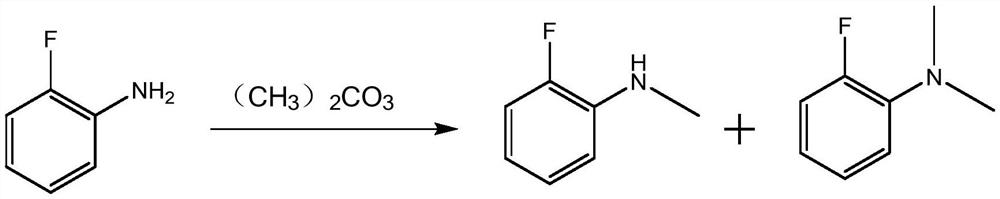
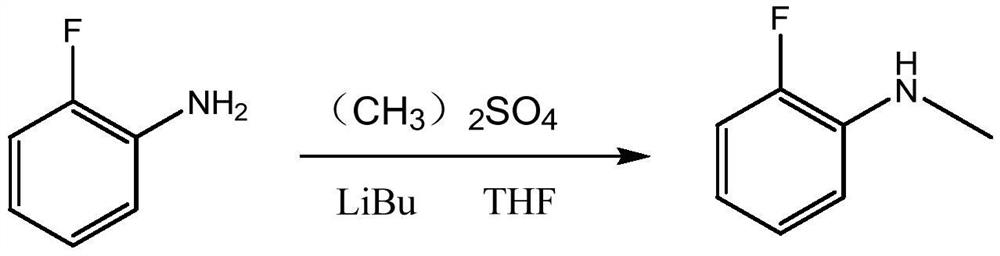
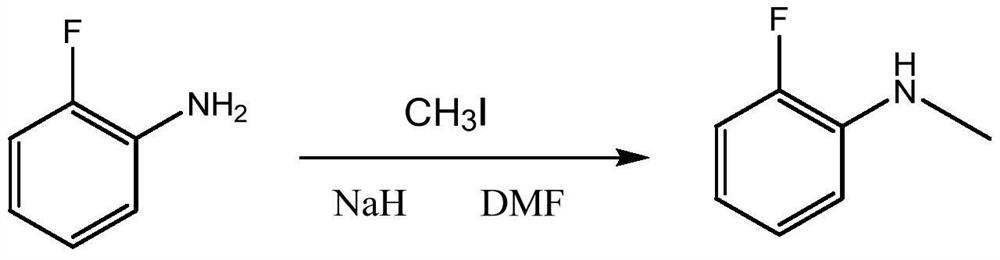
Synthesis method of N-methyl o-fluoroaniline
A technology for the synthesis of methyl o-fluoroaniline, which is applied in the preparation of pharmaceutical intermediates and the field of pesticides. It can solve the problems of increasing tail gas treatment costs and high equipment requirements, and achieve the effects of large-scale industrial application prospects, low raw material costs, and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Amidation reaction:
[0036] Put 111g (1mol) of o-fluoroaniline, 400g of toluene, and 78.5g of formic acid (concentration 88%, 1.5mol) into the reaction flask, and heat up to 88°C. At this time, water begins to come out, and the temperature continues to rise while ensuring that the material is not flushed To 105°C-110°C, heat preservation and dehydration, the total dehydration time is 6h; after the reaction, the reaction solution is cooled to 30°C, washed with water until the pH value is 6-7, separated, and the organic phase is decompressed to distill the toluene to obtain N 136.6 g of -(2-fluorophenyl) formamide, the purity measured by liquid phase was 99%, and the yield was 98.2%.
[0037]
[0038] Methylation reaction:
[0039] Put 139g (1mol) of N-(2-fluorophenyl)formamide, 360g (4mol) of dimethyl carbonate, and 6.9g (0.05mol) of potassium carbonate into an autoclave (with a condenser), raise the temperature to 130°C, and press Gradually rise to 0.6MPa, slowly ...
Embodiment 2
[0045] Amidation reaction:
[0046] Put 111g (1mol) o-fluoroaniline, 400g toluene, 68g formic acid (concentration 88%, 1.3mol) into the reaction flask, heat up to 88°C, water begins to come out, continue to heat up to 105°C ~ 110°C, heat preservation and dehydration, the total The dehydration time is 6 hours. After the reaction, the temperature of the reaction solution is lowered to 30°C, washed with water until the pH value is 6-7, separated into layers, and the toluene is evaporated from the organic phase under reduced pressure to obtain N-(2-fluorophenyl)formamide 136.2 g, the purity measured by the liquid phase is 99%, and the yield is 97.9%.
[0047] Methylation reaction:
[0048]Put 139g (1mol) of N-(2-fluorophenyl)formamide, 270g (3mol) of dimethyl carbonate, and 6.9g (0.05mol) of potassium carbonate into an autoclave (with a condenser), raise the temperature to 130°C, and press Gradually rise to 0.6MPa, slowly evacuate to keep the pressure stable at 0.4MPa-0.6MPa, an...
Embodiment 3
[0052] amidation reaction
[0053] With embodiment 1.
[0054] methylation reaction
[0055] With embodiment 1.
[0056] Hydrolysis reaction
[0057] Put 830g of water into the reaction flask, add 160g (1.6mol) of 98% sulfuric acid dropwise, add 153g (1mol) of N-methyl-N-(2-fluorophenyl)formamide, heat up to 80°C, and keep it warm under stirring 5 hours. After the reaction, the temperature of the reaction solution was lowered to 30°C, and 10% sodium hydroxide was added dropwise until the pH value was 7 to 8. After standing, the layers were separated, and the fraction at 85 to 90°C (20mmHg) was collected under negative pressure to obtain N-methyl-ortho Fluoroaniline 115g, the purity measured by the gas phase is 99.2%, and the molar yield is 91.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com