Purification method of carbetocin
A carbetocin and purification method technology, which is applied in the field of carbetocin purification, can solve the problems of difficult separation to obtain high-purity products, complex purification and refining process, and high cost, and shorten the purification process route, improvement of purification efficiency, production cycle and the effect of cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
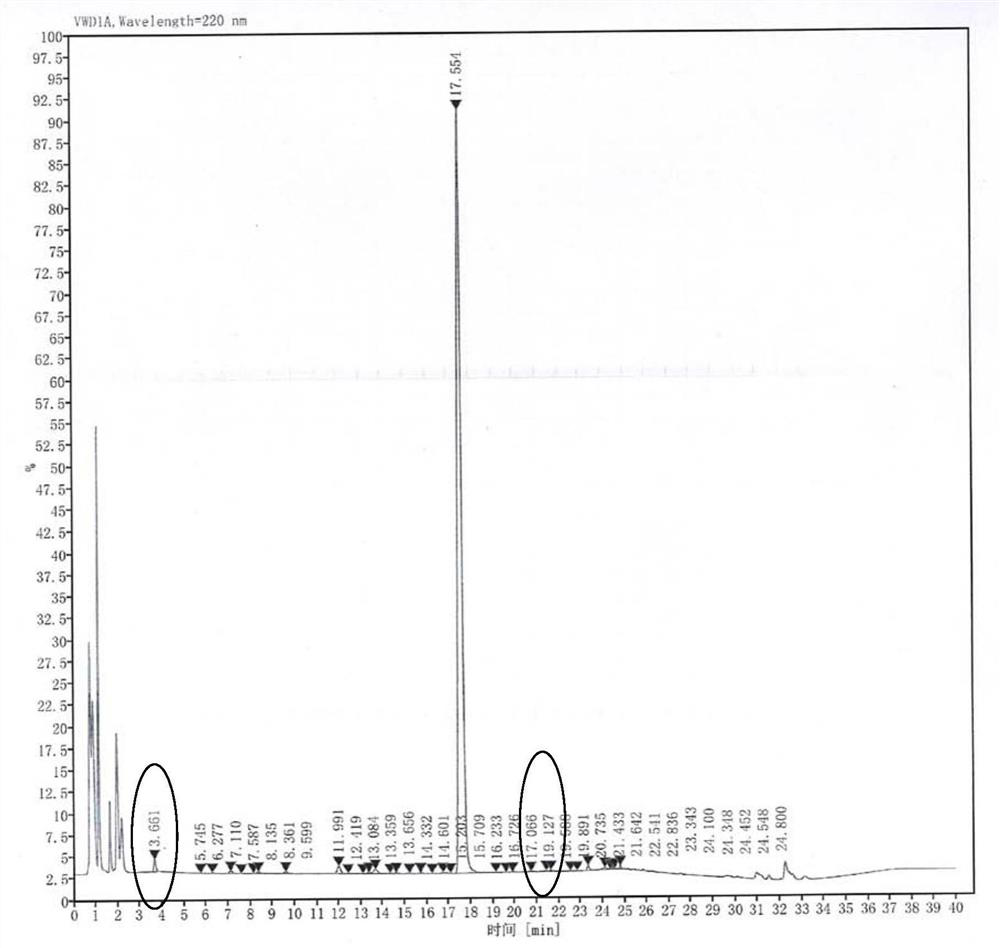
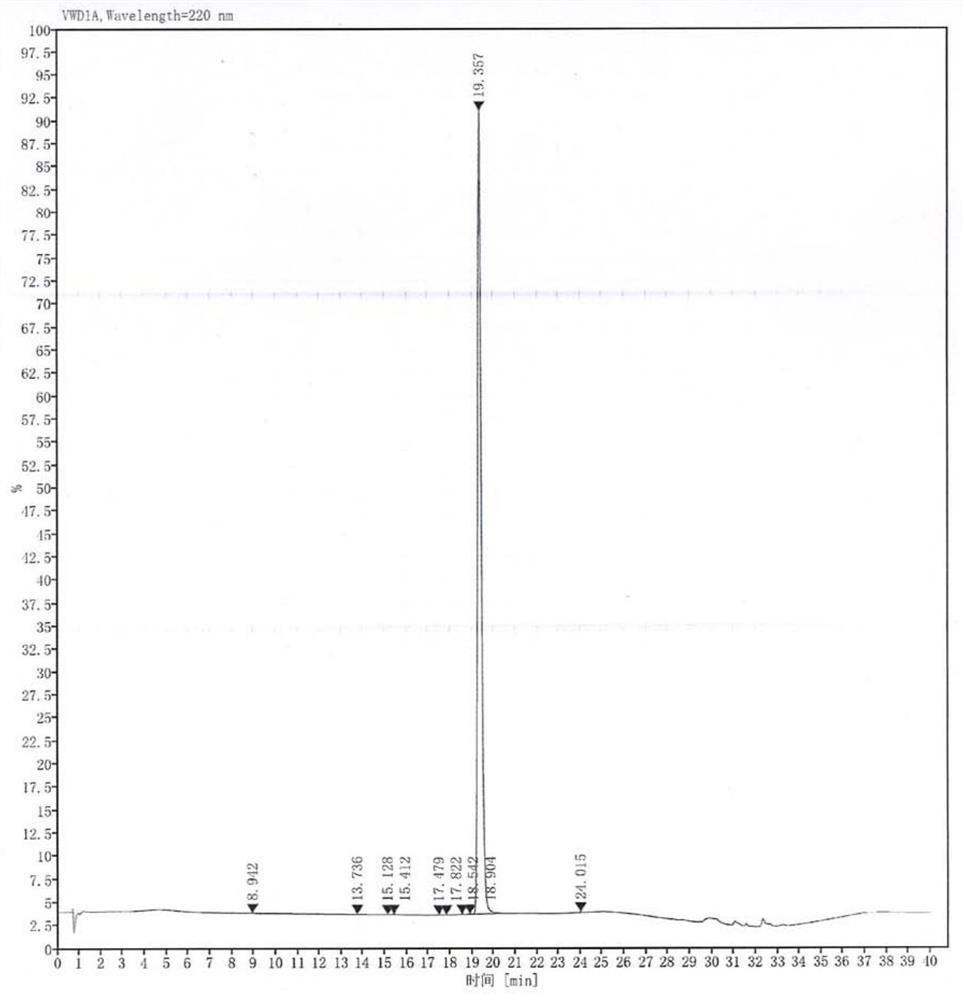
Image
Examples
Embodiment 1
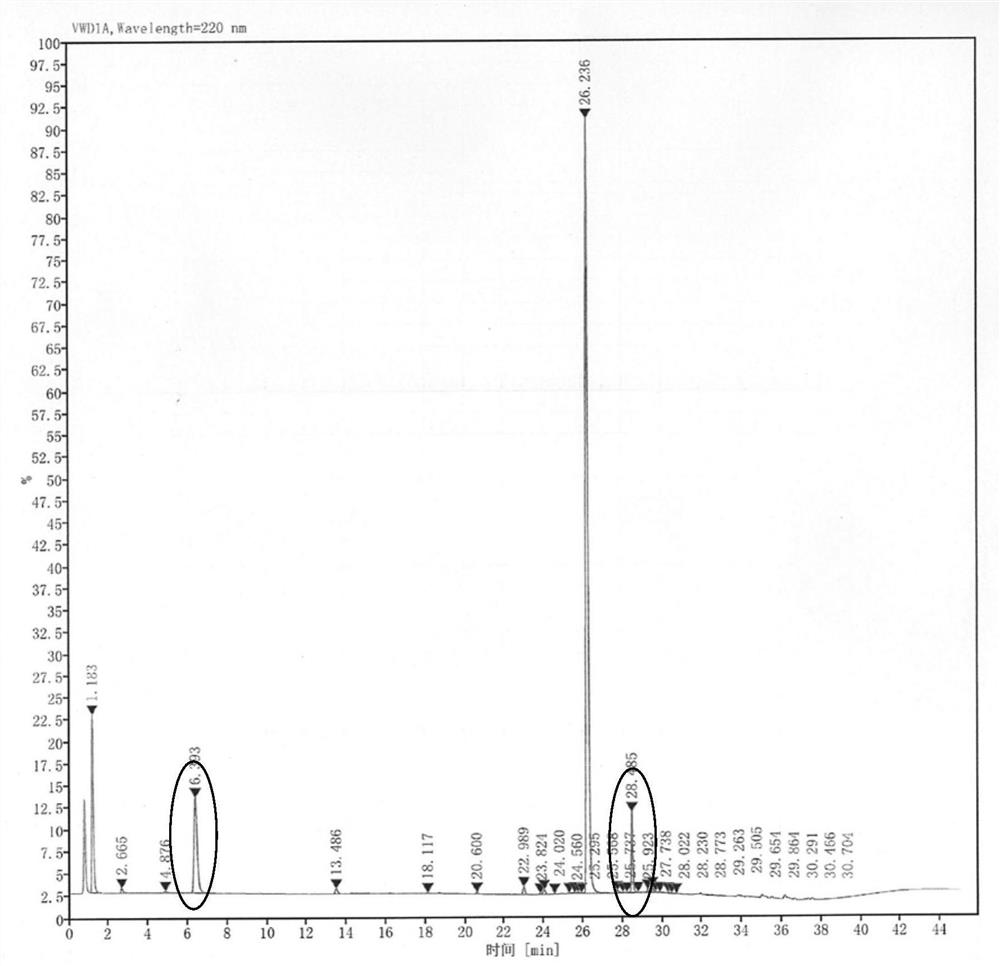
[0052] (1) Sample treatment: The crude carbetocin peptide with a chromatographic purity of 60% to 70% was prepared by solid-phase synthesis, and 3L of a mixed solution of purified water-acetonitrile (1:1) was used, according to 15g to 20g / L The concentration is dissolved, stirred, and after complete dissolution, filter with a 0.45um water-based filter membrane to remove insoluble matter and obtain the filtrate, which is the crude carbetocin solution.
[0053] The HPLC spectrogram of the obtained carbetocin crude product solution is as follows figure 1 shown.
[0054] (2) Salt precipitation:
[0055] First, add trifluoroacetic acid solution (1.0%) to adjust the pH value of the crude product solution to 2.0-2.5, and place it at 2-8°C for refrigerated storage for 2-4h, filter off the gray precipitate; then adjust the pH value of the crude product solution with alkali (ammonia water) solution The value is 7.5-8.5, and stored at 2-8°C for 2-4 hours, filtered to remove the precipi...
Embodiment 2
[0065] (1) Sample processing:
[0066] 55g of carbetocin crude peptide with a chromatographic purity of 60% was obtained by solid-phase synthesis, dissolved in 3L of purified water-acetonitrile (1:1) mixed solution according to the concentration of 15g-20g / L, stirred, and completely dissolved Afterwards, filter with a 0.45um water-based filter membrane to remove insoluble matter and obtain the filtrate, which is the crude carbetocin solution.
[0067] (2) Salt precipitation:
[0068] First add TFA solution (0.5%) to adjust the pH value of the crude product solution to 2.0-2.5, and place it at 2-8°C for refrigerated storage for 2-4h, filter off the gray precipitate; then use alkali (ammonia) solution to adjust the pH value of the crude product solution. 7.5-8.5, and stored at 2-8°C for 2-4 hours, filtered to remove the precipitate; then use TFA solution (0.5%) to adjust the pH value of the crude product solution to 3.5-4.5, and store at 2-8°C for 2 Filter after -4h.
[0069]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com