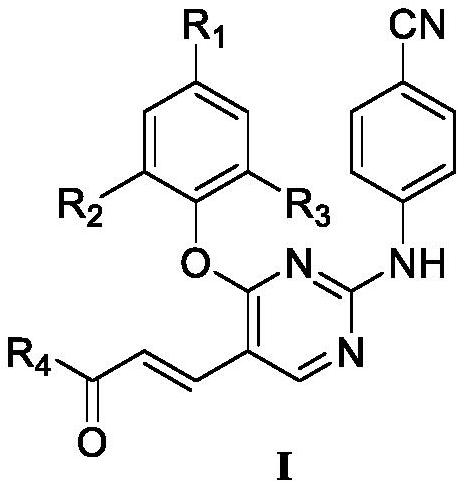
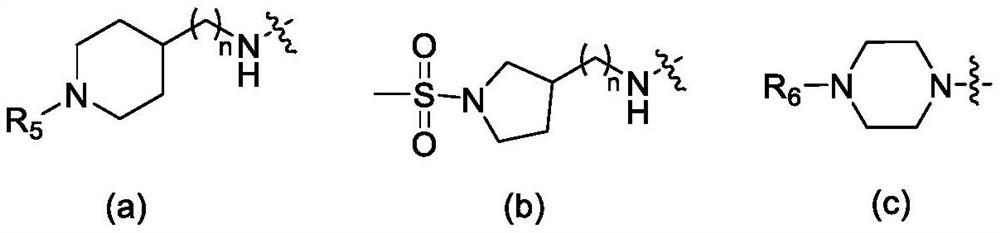
Diarylpyrimidine HIV-1 reverse transcriptase inhibitor containing trans-double bonds, and preparation method and application thereof
A technology of diarylpyrimidines and reverse transcriptase inhibition, which is applied in the fields of organic compound synthesis and medical application, can solve the problems of low bioavailability, poor water solubility, cross-resistance and the like, and achieves the effect of high application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Ethyl (E)-3-(4-(4-cyano-2,6-dimethylphenoxy)-2-((4-cyanophenyl)amino)pyrimidine-5- base) preparation of ethyl acrylate (Z1)
[0042]
[0043] Dissolve 2,4-dichloropyrimidine (0.10g, 0.67mmol) and 4-hydroxy-3,5-dimethylbenzonitrile (0.12g, 0.8mmol) in 30mL DMF, add K 2 CO 3 (0.11g, 0.8mmol), stirred at room temperature for 6h, and detected the reaction by TLC. After the reaction, extract with dichloromethane (10mL×3), combine several layers, wash with saturated brine (30mL) once, anhydrous Na 2 SO 4 Dry for 5h, filter and mix the sample. Column separation and recrystallization from ethyl acetate / petroleum ether gave white solid I-2. Yield: 80%; melting point 126-128°C.
[0044] Palladium acetate (0.004g, 0.0019mmmol) and 4,5-bisdiphenylphosphine-9,9-dimethylxanthene (0.009g, 0.0019mmol) were dissolved in dioxane (15mL), room temperature After stirring and activating for 15 min, compound I-2 (0.1 g, 0.38 mmol) and cesium carbonate (4.89 g, 0.49 mmol) ...
Embodiment 2
[0049] Example 2: (E)-3-(4-(4-cyano-2,6-dimethylphenoxy)-2-((4-cyanophenyl)amino)pyrimidin-5-yl) Preparation of acrylamide (Z2)
[0050]
[0051] Dissolve I-6 (0.15g, 0.36mmol) in dichloromethane (10mL), add oxalyl chloride (100μL, 1.16mmol), add a drop of DMF as catalyst, stir overnight at room temperature, evaporate to dryness under reduced pressure, add 30mL ammonia water , heated to reflux at 50°C for 6h, and the reaction was detected by TLC. After the reaction, the filter residue was obtained by filtration and dried. Dissolve the dried product (0.2g) in 10mL of dichloromethane, add 10mL of ammonia water, reflux at 45°C for 6h, mix the sample, separate by column, and recrystallize from ethyl acetate / petroleum ether to obtain the final product (E)-3-(4- (4-cyano-2,6-dimethylphenoxy)-2-((4-cyanophenyl)amino)pyrimidin-5-yl)acrylamide.
[0052] The product is a white solid, yield 50.1%, melting point: 226-228°C.
[0053] 1 H NMR (400MHz, DMSO-d 6 ):δ10.40(s,1H,NH),8.7...
Embodiment 3
[0054] Embodiment 3: the preparation of target compound (Z3~Z10, Z20, Z21)
[0055] I-5 (0.1g, 0.24mmol) and 2-(7-benzotriazole oxide)-N,N,N',N'-tetramethyluronium hexafluorophosphate (0.11g, 0.288mmol) Dissolve in DMF (10mL), activate in ice bath for 15min, add N,N-diisopropylethylamine (121μL, 0.72mmol) and cyclopropylamine (20μL, 0.288mmol) to continue activation for 15min, stir at room temperature for 6h, TLC detection reaction . After the reaction was complete, it was concentrated and evaporated to dryness. Add 40mL of water, extract with dichloromethane (20mL×3), combine several layers, wash with saturated brine (30mL) once, anhydrous Na 2 SO 4 Dry for 5h, filter, concentrate, and mix samples. After column separation and ethyl acetate / petroleum ether recrystallization, the target compound Z3 was obtained.
[0056]
[0057] The product is a white solid, yield 78.6%, melting point: 254-255°C.
[0058] 1 H NMR (400MHz, DMSO-d 6 ):δ10.41(s,1H,NH),8.75(s,1H,C 6 -p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com