Positive electrode material and manufacturing method therefor, battery using positive electrode material and manufacturing method therefor, and electronic equipment using battery
A technology of cathode material and manufacturing method, applied in the field of electronic equipment, capable of solving problems such as insufficient energy density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0245]
[0246] Lithium carbonate (2.96g), cobalt oxalate dihydrate (7.32g) and diammonium hydrogen phosphate (10.56g) were placed in a planetary ball mill vessel. Then, the planetary ball mill container is placed on the ball mill device, and the ball mill device is driven to mix the raw materials. The resulting mixture was calcined at 600° C. for 12 hours under an argon atmosphere. The obtained calcined product was pulverized by a planetary ball mill to obtain amorphous Li 2 CoP 2 o 7 . It was further annealed at 500°C for 30 minutes under an argon atmosphere, thereby obtaining Li 2 CoP 2 o 7 .
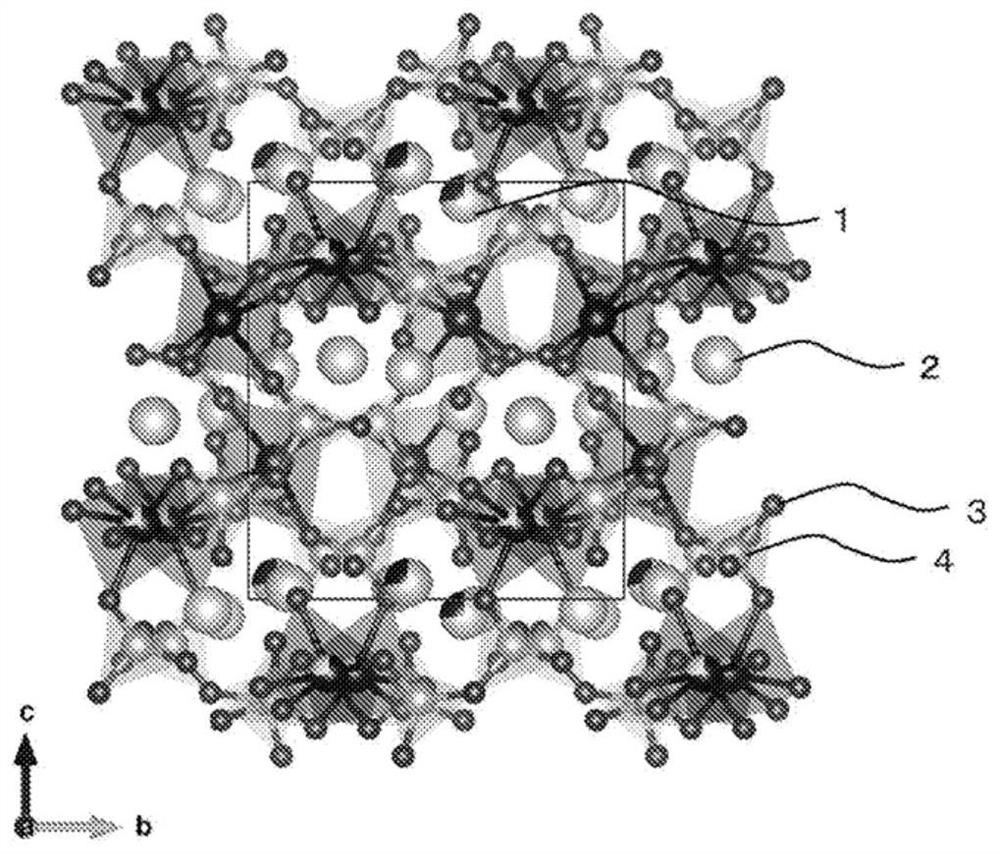
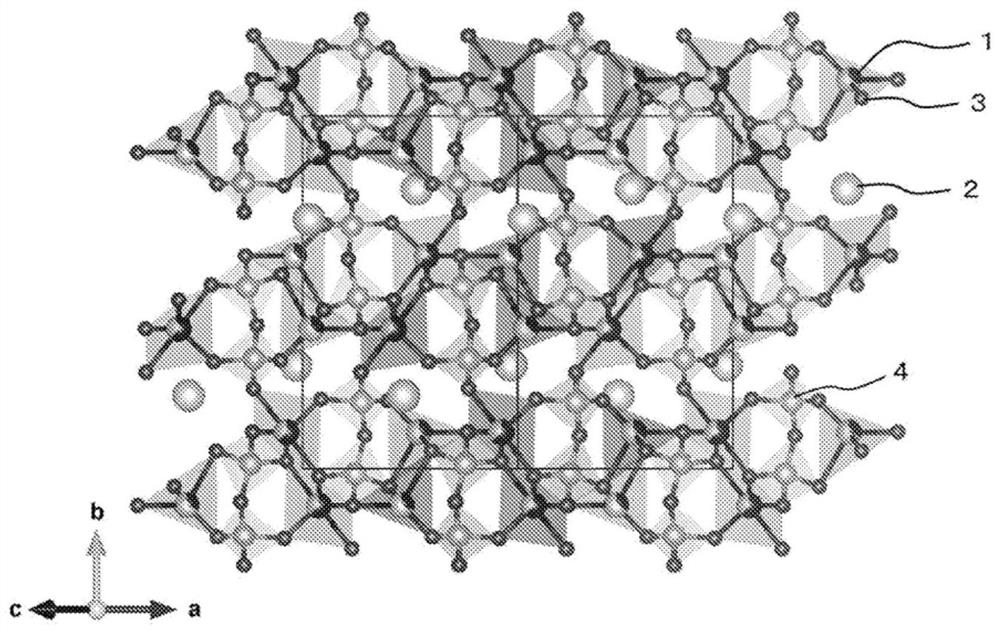
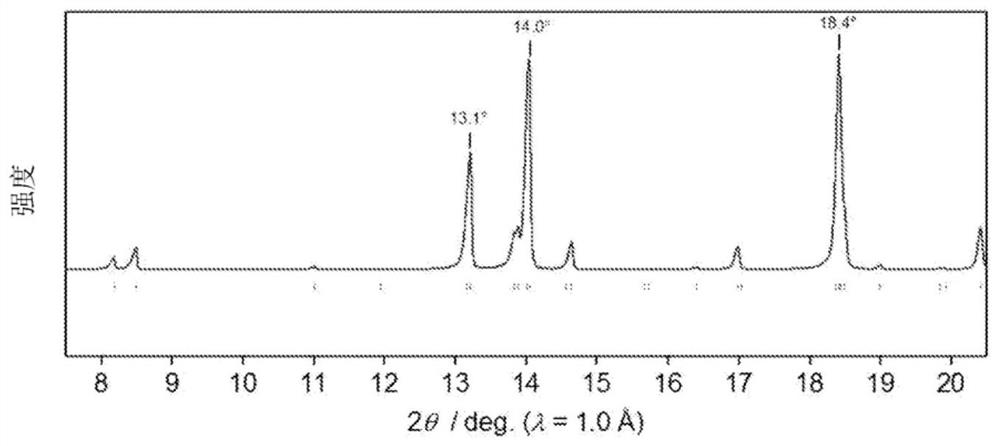
[0247] The wavelength of the resulting cathode material The radiation light diffraction (X-ray diffraction) spectrum of Figure 5 (A). In the obtained spectrum, there are strong diffraction peaks at 2θ=13.1°, 14.0°, and 18.4°. Using this spectrum for Li 2 CoP 2 o 7 According to the analysis of the crystal structure, it can be seen that the crystal phase is monoclinic...
Embodiment 2
[0254] Lithium carbonate (3.55g), cobalt oxalate dihydrate (5.86g) and diammonium hydrogen phosphate (10.56g) were placed in a planetary ball mill vessel. Then, the planetary ball mill container is placed on the ball mill device, and the ball mill device is driven to mix the raw materials. The resulting mixture was calcined at 600° C. for 12 hours under an argon atmosphere. The obtained calcined product was pulverized by a planetary ball mill to obtain amorphous Li 2.4 co 0.8 P 2 o 7 . It was further annealed at 500°C for 30 minutes under an argon atmosphere, thereby obtaining Li 2.4 co 0.8 P 2 o 7 .
[0255] The wavelength of the obtained positive electrode material Radiation light diffraction (X-ray diffraction) measurement. As a result, it can be seen that the Figure 5 The same diffraction pattern in (A) is the same crystal lattice as that of the positive electrode material of Example 1.
Embodiment 3
[0257] Lithium carbonate (2.37g), cobalt oxalate dihydrate (8.78g) and diammonium hydrogen phosphate (10.56g) were placed in a planetary ball mill vessel. Then, the planetary ball mill container is placed on the ball mill device, and the ball mill device is driven to mix the raw materials. The resulting mixture was calcined at 600° C. for 12 hours under an argon atmosphere. The obtained calcined product was pulverized by a planetary ball mill to obtain amorphous Li 1.6 co 1.2 P 2 o 7 . It was further annealed at 500 °C for 30 min under an argon atmosphere, thereby obtaining Li with a crystal structure 1.6 co 1.2 P 2 o 7 .
[0258] The wavelength of the obtained positive electrode material Radiation light diffraction (X-ray diffraction) measurement. As a result, it can be seen that the Figure 5 The same diffraction pattern in (A) is the same crystal lattice as that of the positive electrode material of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge capacity | aaaaa | aaaaa |
| Discharge capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap