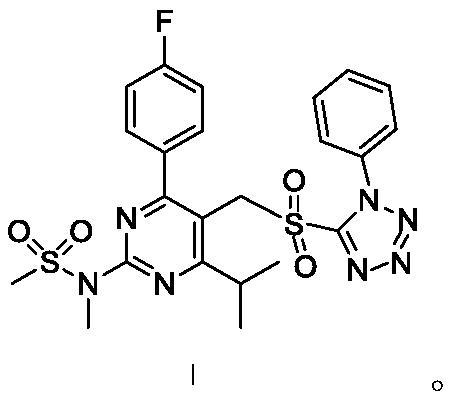
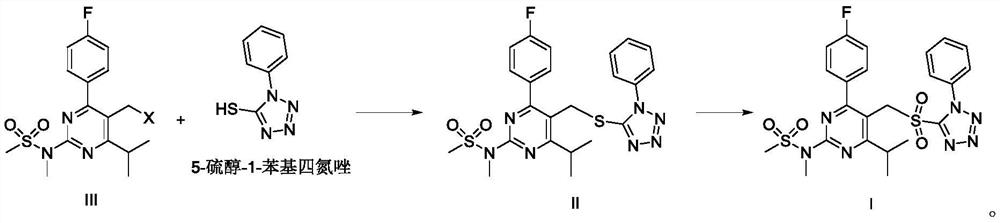
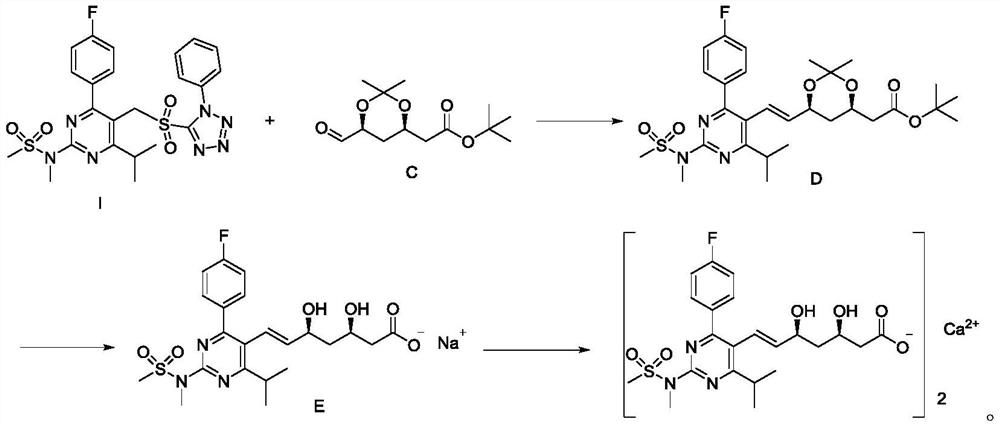
Rosuvastatin calcium intermediate compound
A technology for rosuvastatin calcium and a compound, applied in the field of pharmaceutical synthesis, can solve the problems of low yield, high impurity content of rosuvastatin calcium Z-isomer, low reaction selectivity, etc. Good water solubility and high HPLC purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] The preparation of embodiment 1 compound I
[0079] (1) Add 507.6g of compound III (X=OTs), 196.0g of 5-thiol-1-phenyltetrazolium, 155.1g of diisopropylethylamine and 2.5L of toluene into a three-necked flask, and control the temperature to 0 ~5°C, stir for 3~5 hours, TLC detection, after the reaction is complete, add 2.0L deionized water, stir, wash, and separate liquids. The organic phase was washed with 500 ml of saturated brine, separated, anhydrous sodium sulfate was added to the organic phase to dry, and the solution of compound II was obtained by filtration.
[0080] (2) Add 122.3 g of urea hydrogen peroxide complex to the solution of compound II, control the temperature at -5-5° C., stir for reaction, and detect by TLC. After the reaction is complete, add 2.0 L of deionized water, wash and separate. Anhydrous sodium sulfate was added to the organic phase, dried, filtered, and concentrated under reduced pressure to obtain 536.3 g of crude compound I.
[0081] (...
Embodiment 2
[0084] The preparation of embodiment 2 compound I
[0085] (1) Add 431.5g of compound III (X=OMs), 267.3g of 5-thiol-1-phenyltetrazolium, 192.3g of triethylamine and 3.4L of dichloromethane into a three-necked flask, and control the temperature at -5 ~0°C, stirred for 3~5h, detected by TLC, after the reaction was complete, added 2.5L deionized water, stirred, washed, and separated. The organic phase was washed with 500 ml of saturated brine, separated, anhydrous sodium sulfate was added to the organic phase to dry, and the solution of compound II was obtained by filtration.
[0086] (2) Add 91.3 g of peroxyacetic acid to the solution of compound II, control the temperature at -15 to -10° C., stir for reaction, and detect by TLC. After the reaction is complete, add 2.0 L of deionized water to wash and separate the liquid. Anhydrous sodium sulfate was added to the organic phase, dried, filtered, and concentrated under reduced pressure to obtain 532.7 g of crude compound I.
[...
Embodiment 3
[0090] The preparation of embodiment 3 compound I
[0091] (1) Add 371.9g of compound III (X=Cl), 187.1g of 5-thiol-1-phenyltetrazolium, 87.0g of pyridine and 3.4L of chloroform into a three-necked flask, control the temperature at 5-10°C, and stir React for 3 to 5 hours, TLC detection, after the reaction is complete, add 2.5L of deionized water, stir and wash, and separate liquids. The organic phase was washed with 500 ml of saturated brine, separated, anhydrous sodium sulfate was added to the organic phase to dry, and the solution of compound II was obtained by filtration.
[0092] (2) Add 64.6 g of hydrogen peroxide to the solution of compound II, control the temperature at -10 to -5° C., stir the reaction, and detect it by TLC. After the reaction is complete, add 2.0 L of deionized water to wash and separate the liquid. Anhydrous sodium sulfate was added to the organic phase, dried, filtered, and concentrated under reduced pressure to obtain 530.3 g of crude compound I. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com