2,7-bis(2,4-dimethoxyphenyl)diphenylmethylene fluorene, trimer compound and preparation method and application thereof
A technology of dimethoxyphenyl and dibenzylidene fluorene, which is applied in the field of organic light-emitting materials, can solve problems such as light emission, and achieve the effects of expanding application range, high yield, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
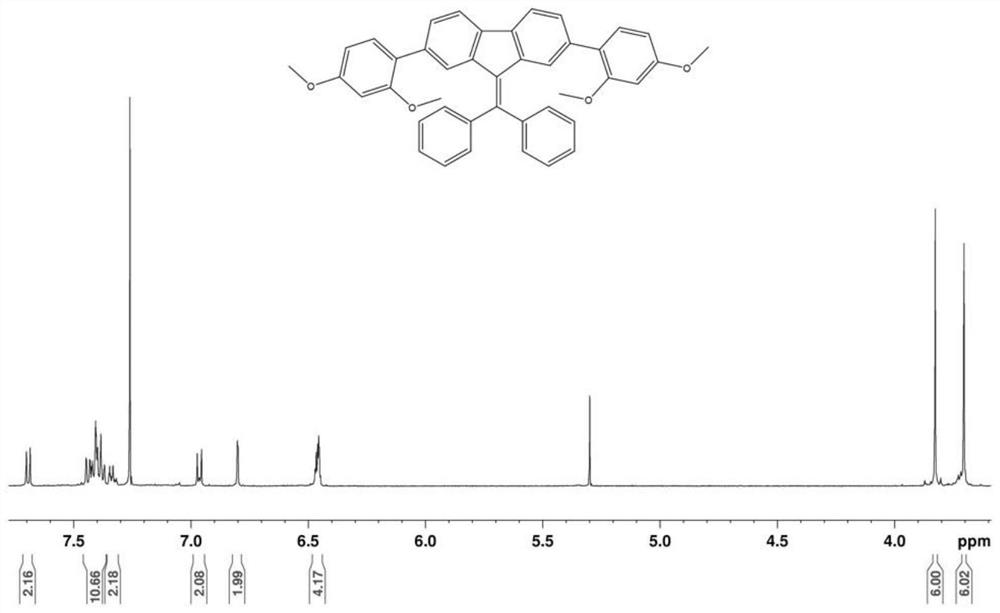
[0042] In this embodiment, a kind of 2,7-bis(2,4-dimethoxyphenyl) dibenzylidene fluorene, the structural formula of its compound is:
[0043]
Embodiment 2
[0044] The preparation method of the present embodiment 2,7-bis(2,4-dimethoxyphenyl) dibenzylidene fluorene, the steps are as follows:
[0045] a. Preparation of intermediate product 2,7-bis(2,4-dimethoxyphenyl)fluorenone:
[0046] Using 2,7-dibromofluorenone and 2,4-dimethoxybiphenylboronic acid as reactant raw materials, 2,7-dibromofluorenone and 2,4-dimethoxybiphenylboronic acid are Dissolve in a solvent with a ratio of 1:2.5, the solvent is a mixed solvent of 1,4-dioxane and water mixed in a volume ratio of 4:1; then add anhydrous sodium carbonate and catalyst [1,1' -bis(diphenylphosphino)ferrocene]palladium dichloride to obtain a reactant solution, the addition of the catalyst [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride The amount is 2% mol according to the total mass of the reactant raw material, and the addition of the anhydrous sodium carbonate is to reflux the reactant solution for 12h according to the 3mol of the total mass of the reactant raw materia...
Embodiment 2
[0055] This embodiment is basically the same as Embodiment 1, especially in that:
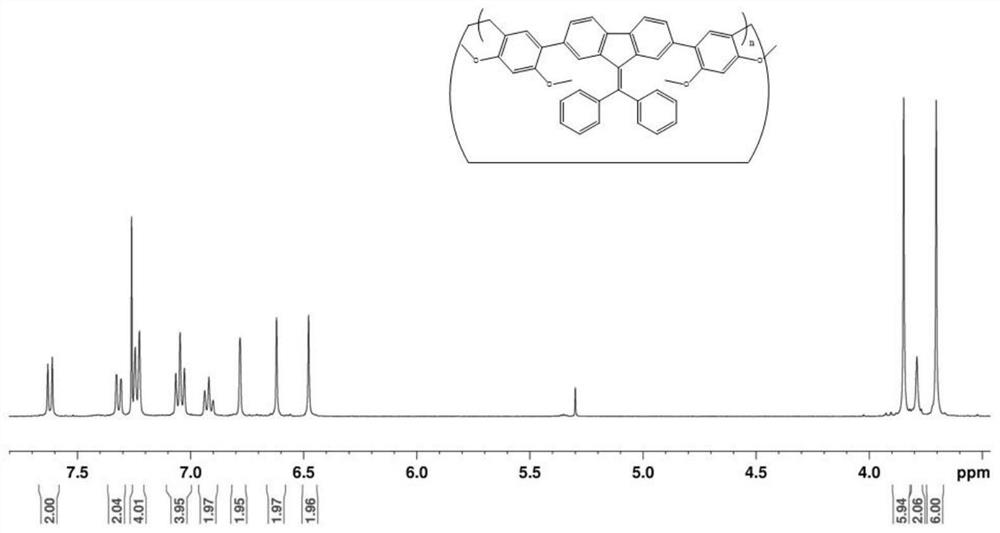
[0056] In this embodiment, a trimer compound, its structural formula is:
[0057]
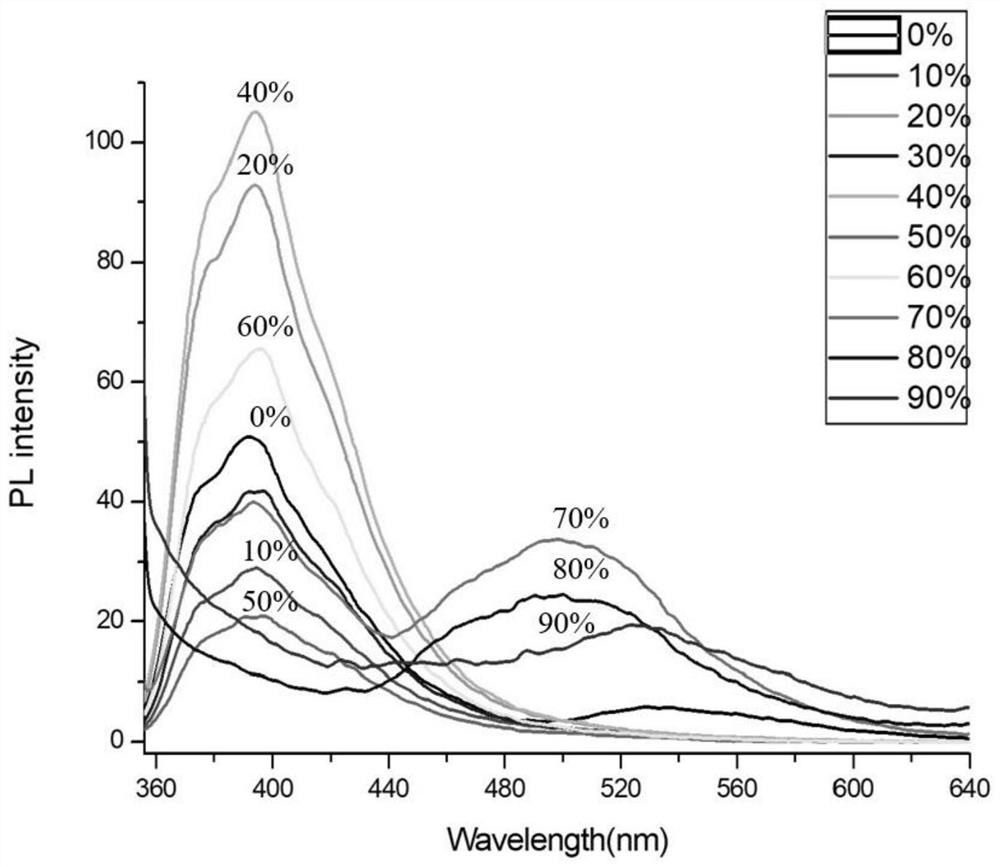
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com