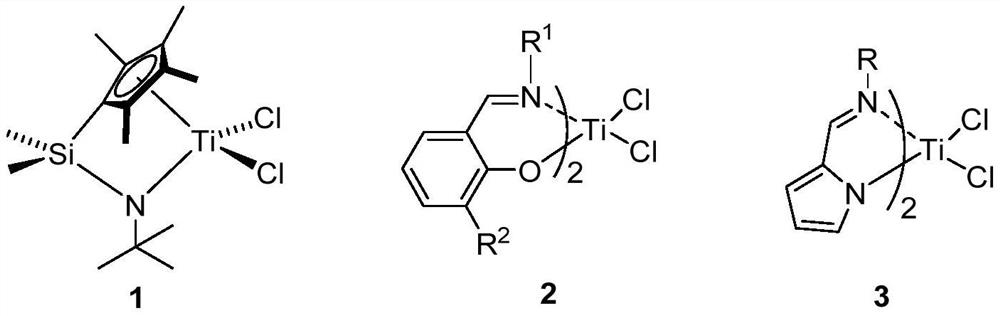
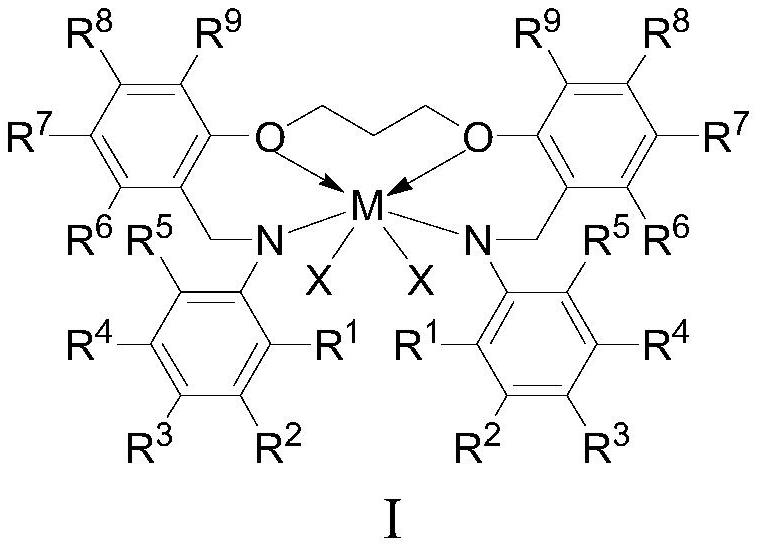
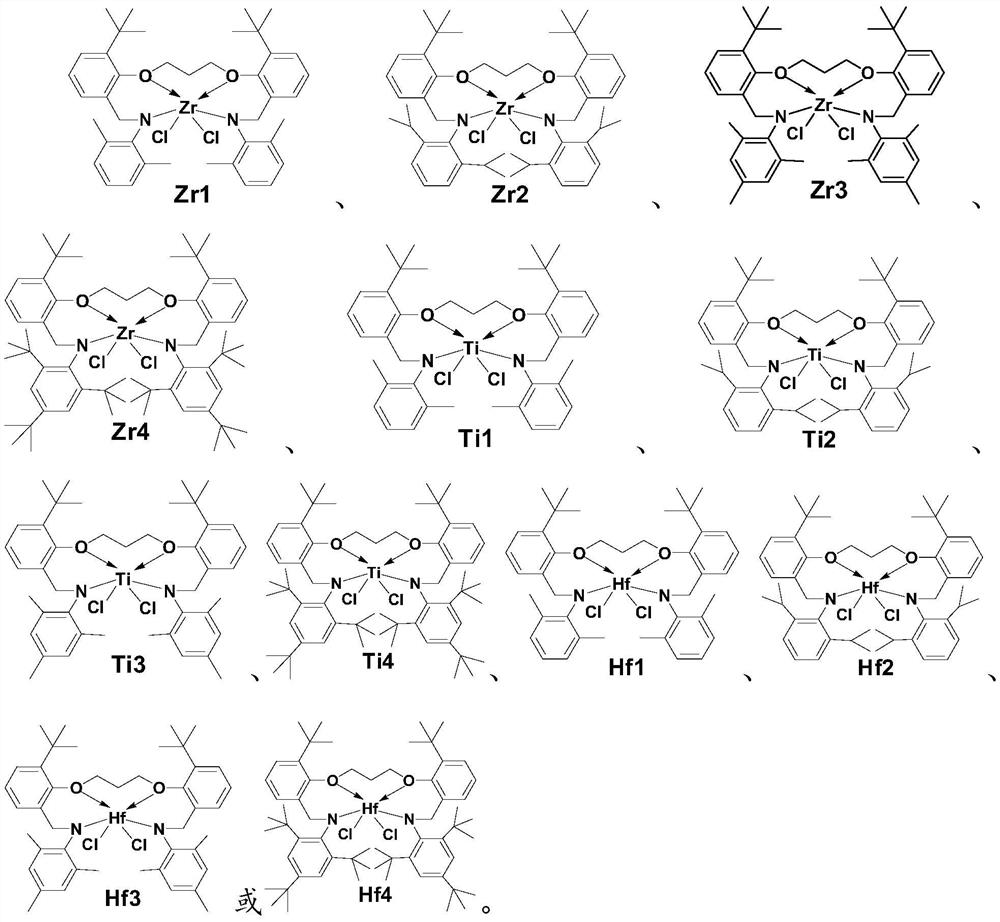
A kind of arylamino ether metal complex, its preparation method and application
A technology of metal complexes and aryl amino ethers, which is applied in the field of homopolymerization of ethylene or copolymerization of ethylene and α-olefins, and can solve the problems of complex synthetic routes and high costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Preparation of compounds represented by formula A1 (R 1 =R 5 =Me;R 2 =R 3 =R 4 =H)
[0095] 3-tert-butylsalicylaldehyde (3.56g, 20mmol) and 2,6-dimethylaniline (2.43g, 20mmol) were respectively added to a 100mL round bottom flask, and 50mL anhydrous methanol and 0.10mL formic acid were added, After heating under reflux for 6 hours, the reaction solution was concentrated and subjected to basic alumina column chromatography (petroleum ether:ethyl acetate=50:1 (v / v)) to obtain 4.82 g of product with a yield of 85.6%. 1 H NMR (CDCl 3 ,400MHz,TMS):δ8.78(s,1H,-CH=N-),7.35(d,J=8.0Hz,1H),7.16–7.14(m,4H),6.88(t,J=8.0Hz , 1H), 5.74(s, 1H, -OH), 2.25(s, 6H), 1.40(s, 9H). 13 C NMR (CDCl 3 ,100MHz,TMS):δ159.0,155.8,152.2,137.1,129.9,129.1,128.0,127.3,126.0,120.0,117.1,34.1,31.6,18.6.Anal.Calcd for C 19 H 23 NO(281.40): C, 81.10; H, 8.24; N, 4.98. Found: C, 80.83; H, 8.61; N, 4.74.
Embodiment 2
[0097] Preparation of compounds represented by formula A2 (R 1 =R 5 = i Pr;R 2=R 3 =R 4 =H)
[0098] The experimental procedure was the same as in Example 1, 3-tert-butylsalicylaldehyde (3.56 g, 20 mmol) was reacted with 2,6-diisopropylaniline (3.55 g, 20 mmol) to obtain 5.66 g of product with a yield of 83.8%. 1 H NMR (CDCl 3 ,400MHz,TMS):δ8.78(s,1H,-CH=N-),7.38-7.35(m,2H),7.15-7.12(m,3H),6.88(t,J=8.0Hz,1H) ,5.74(s,1H,-OH),2.78–2.75(m,2H),1.40(s,9H),1.18(d,J=8.0Hz,12H). 13 C NMR (CDCl 3 ,100MHz,TMS):δ159.0,155.8,146.4,137.1,135.7,129.9,128.0,122.3,121.3,120.0,117.1,34.1,31.6,28.9,23.3.Anal.Calcd for C 23 H 31 NO(337.51): C, 81.85; H, 9.26; N, 4.15. Found: C, 81.53; H, 9.55; N, 4.01.
Embodiment 3
[0100] Preparation of compound represented by formula A3 (R 1 =R 3 =R 5 =Me;R 2 =R 4 =H)
[0101] The experimental procedure was the same as in Example 1, and 3-tert-butylsalicylaldehyde (3.56g, 20mmol) was reacted with 2,4,6-trimethylaniline (2.71g, 20mmol) to obtain 4.85g product with a yield of 82.1% . 1 H NMR (CDCl 3 ,400MHz,TMS):δ8.78(s,1H,-CH=N-),7.35(d,J=8.0Hz,1H),7.15(d,J=8.0Hz,1H),6.98–6.96(m ,3H),5.74(s,1H,-OH),2.34(s,6H),2.18(s,3H),1.40(s,9H). 13 C NMR (CDCl 3 ,100MHz,TMS):δ159.0,155.8,149.2,137.1,129.9,128.2,128.0,125.4,120.0,117.1,34.1,31.6,21.9,18.9.Anal.Calcd for C 20 H 25 NO(295.43): C, 81.31; H, 8.53; N, 4.74. Found: C, 81.00; H, 8.82; N, 4.51.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com