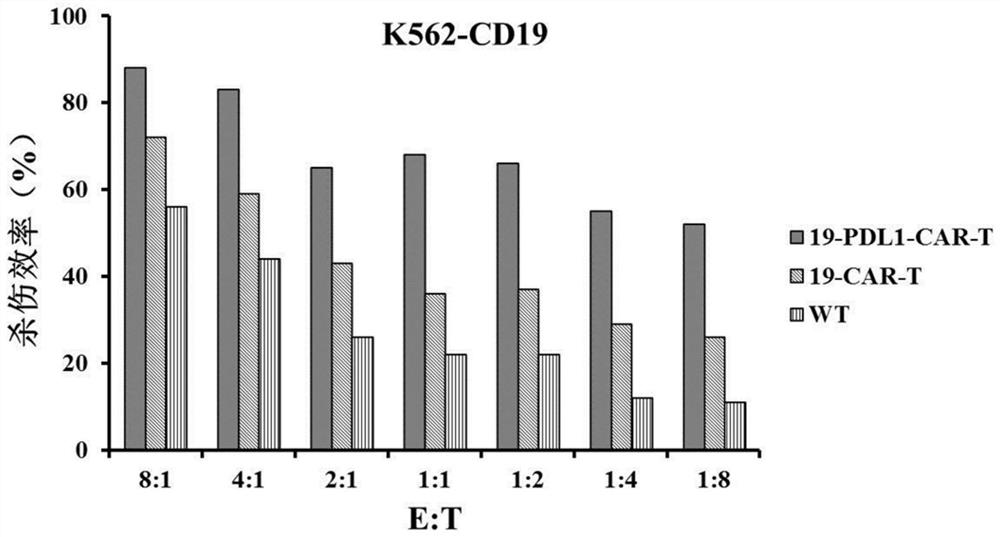
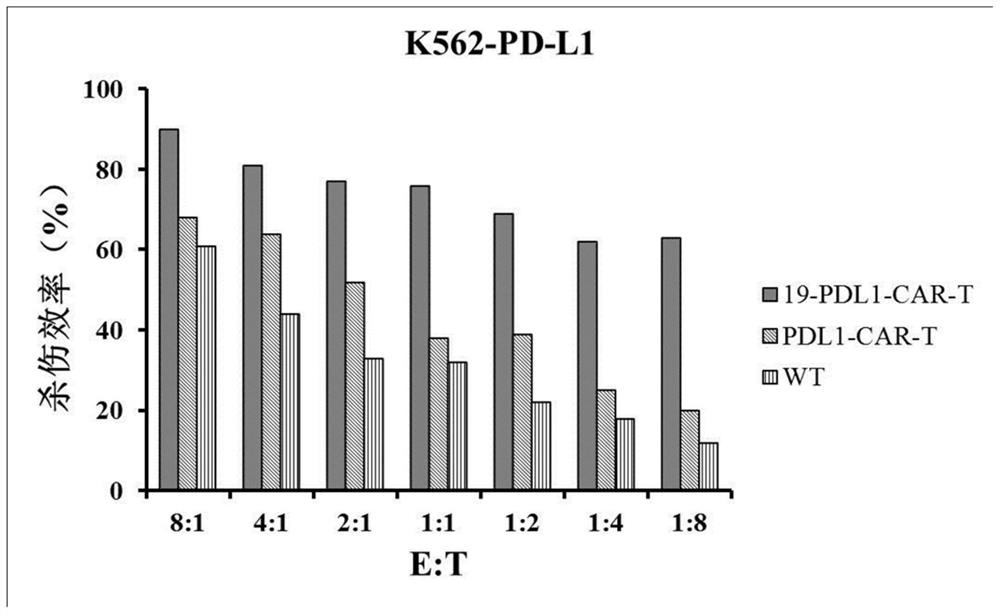
CD19 and PD-L1 double-target chimeric antigen receptor and application thereof
A technology of chimeric antigen receptor and PD-L1, which is applied in the field of biomedicine to alleviate the problem of drug resistance, overcome the mechanism of immune escape, and improve the effect of anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Construction of CAR Molecular Carrier
[0045] In this example, the gene encoding the CD19 and PD-L1 dual-target CAR molecule (the amino acid sequence is shown in SEQ ID NO: 3) was firstly synthesized, and HindIII and BamHI enzyme cleavage sites and their protective bases were added to both ends respectively. base;
[0046] The coding gene was double-digested with restriction endonucleases HindIII and BamHI, and the digested product containing sticky ends was recovered by 1.5% agarose gel electrophoresis, and ligated into the linearized lentiviral expression vector pWPXLd-eGFP. The system is shown in the table 1 shown;
[0047] After incubating at 37°C for 30 minutes, place it quickly on ice for 5 minutes, then add 20 μL of Trans1-T1 competent, let stand for 30 minutes, heat shock at 42°C for 90 seconds, and smear the plate to obtain the recombinant lentiviral vector.
[0048] Table 1
[0049] Reagent Dosage Linearized pWPXLd vector 200ng...
Embodiment 2
[0051] Example 2 lentiviral packaging
[0052] In this example, the lentiviral vector constructed in Example 1 is packaged with lentivirus, and the steps are as follows:
[0053] (1) Culture 293T cells in a 10cm petri dish, the culture medium is DMEM high glucose medium+10% FBS (fetal bovine serum)+1% double antibody (100×penicillin-streptomycin mixed solution);
[0054] (2) When the 293T cell density in the culture dish reaches 80%, replace the medium with DMEM high glucose medium + 1% FBS + 1% double antibody;
[0055] (3) After replacing the medium and culturing for 2 hours, prepare a transfection reagent, take 500 μL opti-DMEM into a 15 mL centrifuge tube, add 7.2 μL of PEI (linear polyethyleneimine) with a concentration of 10 μg / μL, and mix slightly. Stand still for 5 minutes;
[0056] (4) Put 500μL opti-DMEM into a 1.5mL centrifuge tube, take 9μg of recombinant lentiviral vector, 3μg of pMD2.G helper plasmid and 12μg of psPAX, add them to the centrifuge tube, mix well,...
Embodiment 3
[0063] Example 3 T cell activation and lentiviral transfection
[0064] (1) After sorting Pan T cells from umbilical cord blood, count the cells and adjust the concentration to 1×10 6 cells / mL, then add 10 μL of Miltenyi TransAct T cell reagent to each ml of cell suspension, and replace it with fresh medium (IMDM medium + 5% FBS (fetal bovine serum) + 1% double antibody ( 100×penicillin-streptomycin mixed solution)+IL-2);
[0065] (2) After T cells were activated for 48 h, demagnetize the beads, centrifuge at 300 g for 5 min, remove the supernatant, resuspend T cells with fresh medium, add CAR-expressing recombinant lentivirus or blank control lentivirus (MOI=10), and Add 8μg / mL polybrene and 300IU / mL IL-2, place at 37°C, 5% CO 2 incubator cultivation;
[0066] (3) After 24 hours, centrifuge at 300 g for 5 minutes, remove the supernatant, and resuspend the T cells in fresh medium containing 300 IU / mL IL-2 to obtain CAR-T cells.
[0067] The CAR-T cells constructed in this ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com