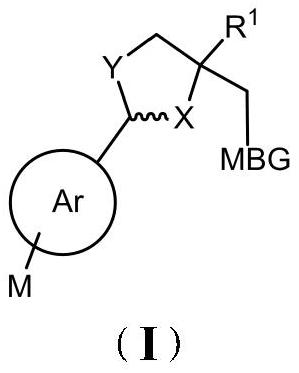
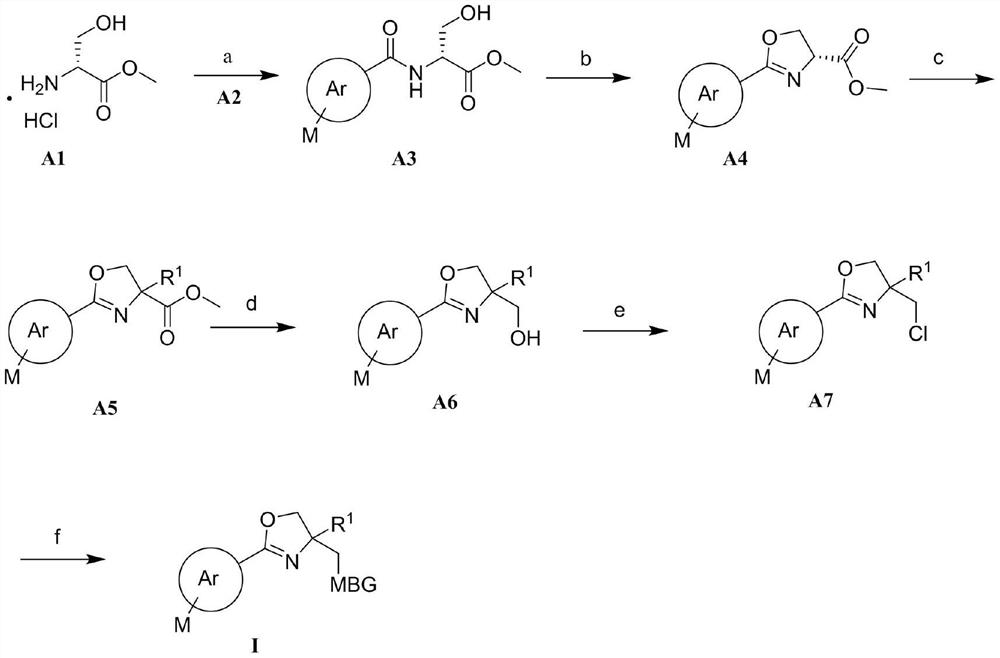
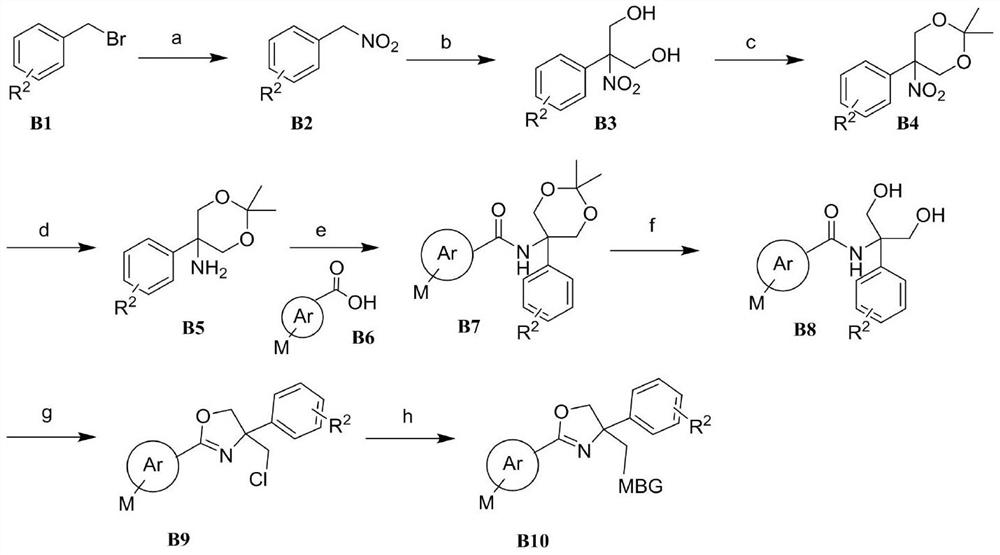
2, 4, 4-trisubstituted dihydrooxazole derivative and application thereof
A kind of dihydrooxazole, unsubstituted technology, applied in the field of drug synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0124] Example 1: 4-((1H-imidazol-1-yl)methyl)-2-([1,1'-biphenyl]-4-yl)-4-methyl-4,5-dihydrooxane azole
[0125]
[0126] In step a: the raw materials 1-1 (1.1 mmol), bibenzoic acid 1-2 (1.1 mmol) and PyBOP (1H-benzotriazol-1-yloxytripyrrolidinyl hexafluorophosphate) (1.2 mmol) were combined ) was added to DMF, stirred at room temperature for 8 h, TLC detected the reaction completion, added water, extracted with ethyl acetate, washed the organic layer with saturated brine, and dried over anhydrous sodium sulfate overnight. The desiccant was filtered off and concentrated under reduced pressure to obtain Intermediate 1-3.
[0127] In step b: intermediate 1-3 (5mmol), SOCl 2 (12.5 mmol) and triethylamine (12.5 mmol) were added to dichloromethane, the reaction was stirred at room temperature, TLC detected the reaction was complete, the organic solvent was evaporated, dissolved in ethyl acetate, washed with water 3 times, the organic layer was washed with saturated NaCl, anhyd...
Embodiment 2
[0133] Example 2: 4-((1H-imidazol-1-yl)methyl)-2-([1,1'-biphenyl]-4-yl)-4-isopropyl-4,5-dihydro oxazole
[0134]
[0135] ESI-MS[M+H] + (m / z): 346.1.
Embodiment 3
[0136] Example 3: 4-((1H-imidazol-1-yl)methyl)-2-([1,1'-biphenyl]-4-yl)-4-cyclopropyl-4,5-dihydro oxazole
[0137]
[0138] ESI-MS[M+H] + (m / z): 344.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com