Brucella ghost strain, Brucella ghost vaccine and preparation method
A Brucella and bacteria shadow technology, applied in biochemical equipment and methods, chemical instruments and methods, and microbial-based methods, can solve human and animal infections, inability to distinguish vaccine immunity from wild virus infection, and virulence Residue and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
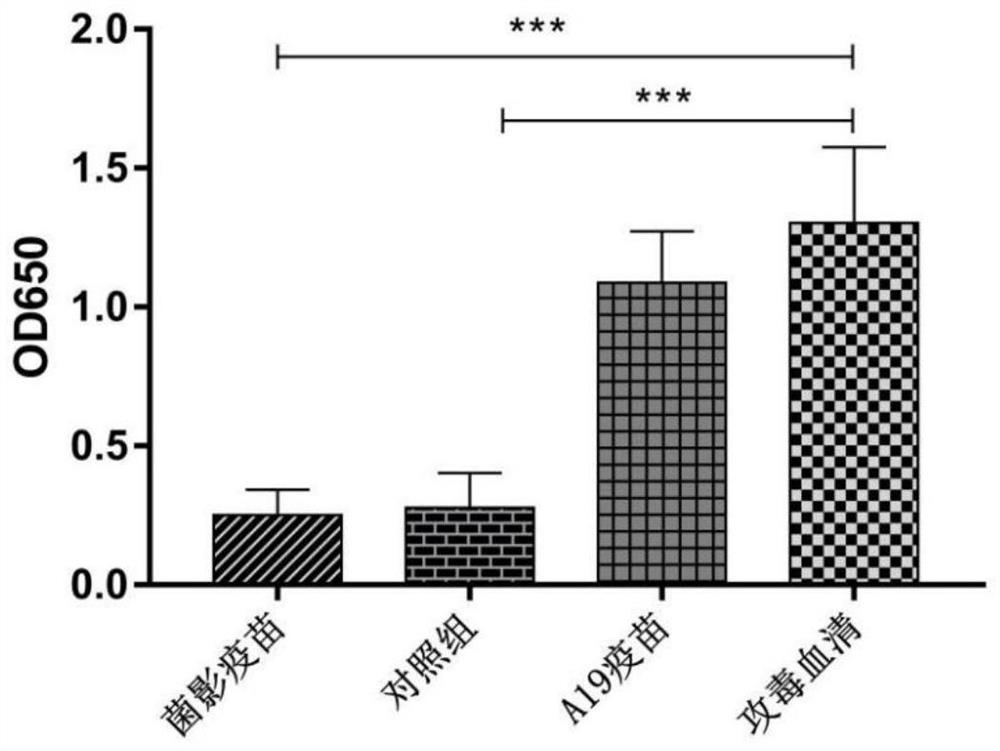
Image
Examples
preparation example Construction
[0060] Also provide the preparation method of above-mentioned Brucella ghost bacterial strain according to second aspect of the present invention, described preparation method comprises:
[0061] The plasmid containing the phage cleavage protein E gene and the Staphylococcus aureus nuclease A gene is transformed into the Brucella strain to obtain the Brucella ghost strain.
[0062] The preparation method of the brucella ghost strain provided by the invention has a simple process, and the brucella ghost strain can be obtained through a conventional transfection operation, has relatively low requirements on equipment and experimenters, and is suitable for popularization and application.
[0063] In some preferred embodiments, the plasmid further includes functional elements.
[0064] Connecting functional elements in the plasmid can provide the corresponding functions to the plasmid. For example, when the temperature control element is selected as the functional element of the p...
Embodiment 1
[0084] Preparation and cultivation of embodiment 1 Brucella bacterium ghost strain
[0085] 1. Preparation of Brucella ghost strains
[0086] 1.1 Construction of the Brucella ghost strain is constructed by transforming the plasmid containing the phage lytic protein E gene, the Staphylococcus aureus nuclease A gene (SNA), and the temperature control element into the A19 strain by electroporation.
[0087] 1.2 Phage E gene amplification and cloning
[0088] 1.2.1 E gene amplification
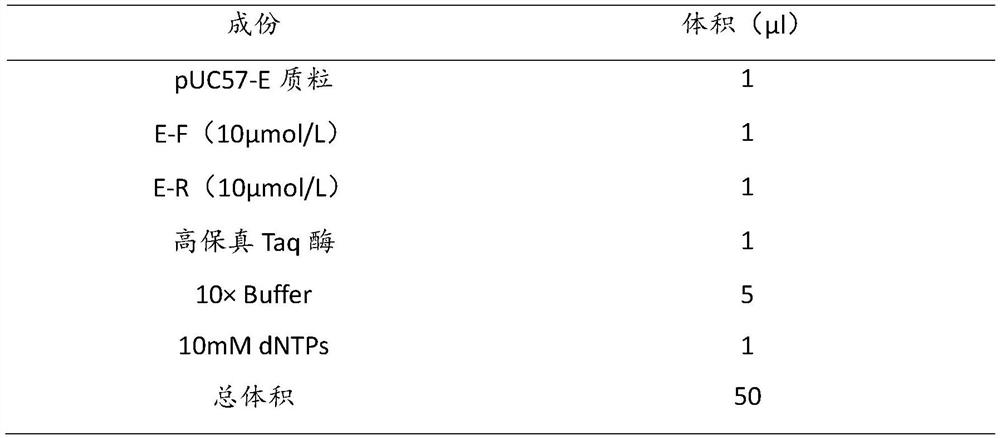
[0089]Using the pUC57-E plasmid as a template, use the E gene amplification primers E-F, E-R for amplification. The reaction conditions are: 94°C pre-denaturation for 5 minutes, 94°C denaturation for 30 seconds, 56°C annealing for 30 seconds, and 72°C extension for 30 seconds. 30 cycles, 72°C extension for 10 minutes. A 50 μl reaction system is used, and the specific components are as follows:
[0090] E gene amplification PCR reaction system table
[0091]
[0092] 1.2.2 E gene cloning
...
Embodiment 2
[0119] The preparation of embodiment 2 Brucella ghost vaccines
[0120] 1. Choose 201 as vaccine adjuvant
[0121] Using the Brucella ghost inactivated antigen provided in Example 1 of the present invention and 201 adjuvant as raw materials, the Brucella ghost vaccine was prepared by emulsification.
[0122] Among them, the antigen content is 300-600 CFU of pre-inactivated Brucella ghost antigen per head, and the mass ratio of antigen and adjuvant is 1:1.
[0123] The emulsification conditions are: temperature 30-34°C, 300-500 rpm, time 10-15min.
[0124] 2. Select 206 as vaccine adjuvant
[0125] Using the Brucella ghost inactivated antigen provided in Example 1 of the present invention and 206 adjuvant as raw materials, the Brucella ghost vaccine was prepared by emulsification.
[0126] Among them, the antigen content is 300-600 CFU of pre-inactivated Brucella ghost antigen per head, and the mass ratio of antigen and adjuvant is 1:1.
[0127] The emulsification condition...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com