Octahydrolycopene dehydrogenase mutant and application thereof
A technology of phytoene and dehydrogenase, which is applied in the field of biosynthesis and can solve problems such as structural analysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 Determines the mutation site that affects the catalytic function of BtCrtI
[0031] In the previous study (CN201910308068), the BtCrtI mutants in the two mutant strains yCC08 and yCC09 we screened contained two point mutations, namely H136R&Y160F; H453R&N576S. In order to determine the mutation sites that affect the catalytic function of BtCrtI, and to explore the synergy between different mutation points, we constructed four single-point mutant strains and nine combined mutant strains according to the method in the patent (CN201910308068). The bacterial numbers and corresponding CrtI mutant types are listed in Table 1.
[0032] Table 1
[0033]
[0034]
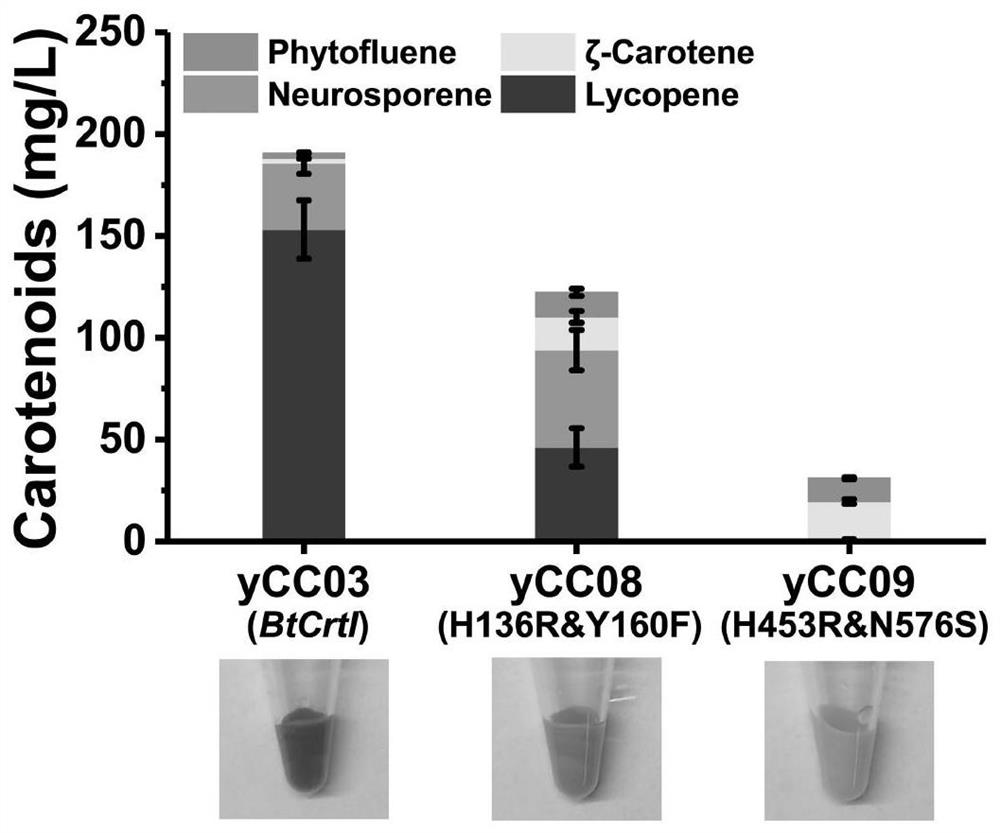
[0035] Shake flask fermentation according to the method in the patent (CN201910308068), the result is as follows figure 1 , figure 2 , as shown in Table 2:
[0036] in conclusion:
[0037] 1. H136R and H453R are the key mutation sites affecting the dehydrogenation function of BtCrtI mutants in...
Embodiment 2
[0043] Example 2 The second round of error-prone PCR mutation screening for H136R and H453R
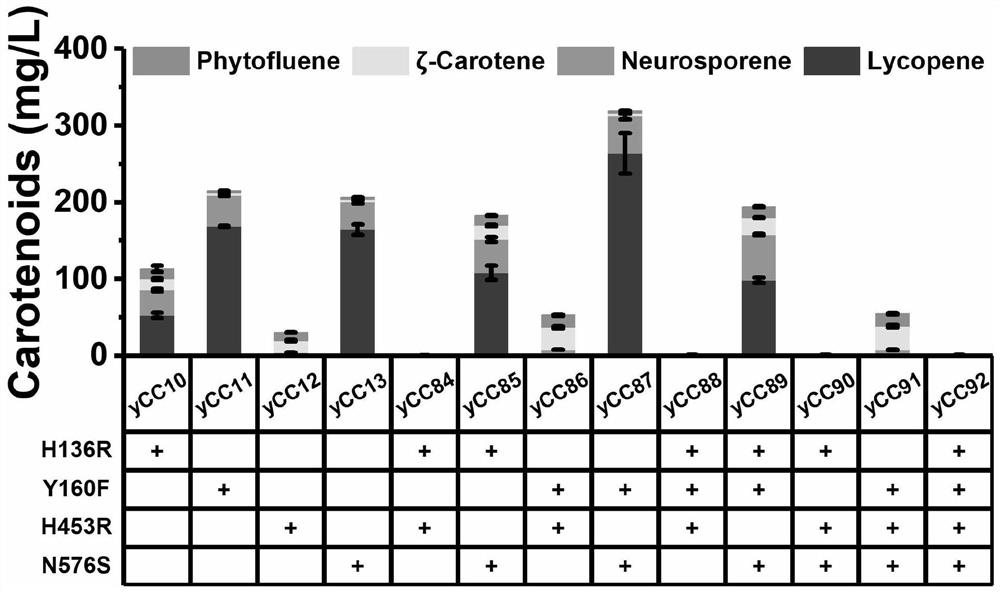
[0044] In order to obtain CrtI mutants with better performance, we performed the second round of error-prone PCR on the basis of H136R and H453R determined by the first round of error-prone PCR. The mutant strains screened are shown in Table 3:
[0045] table 3
[0046]
[0047]
[0048] Shake flask fermentation results are as follows image 3 Shown:
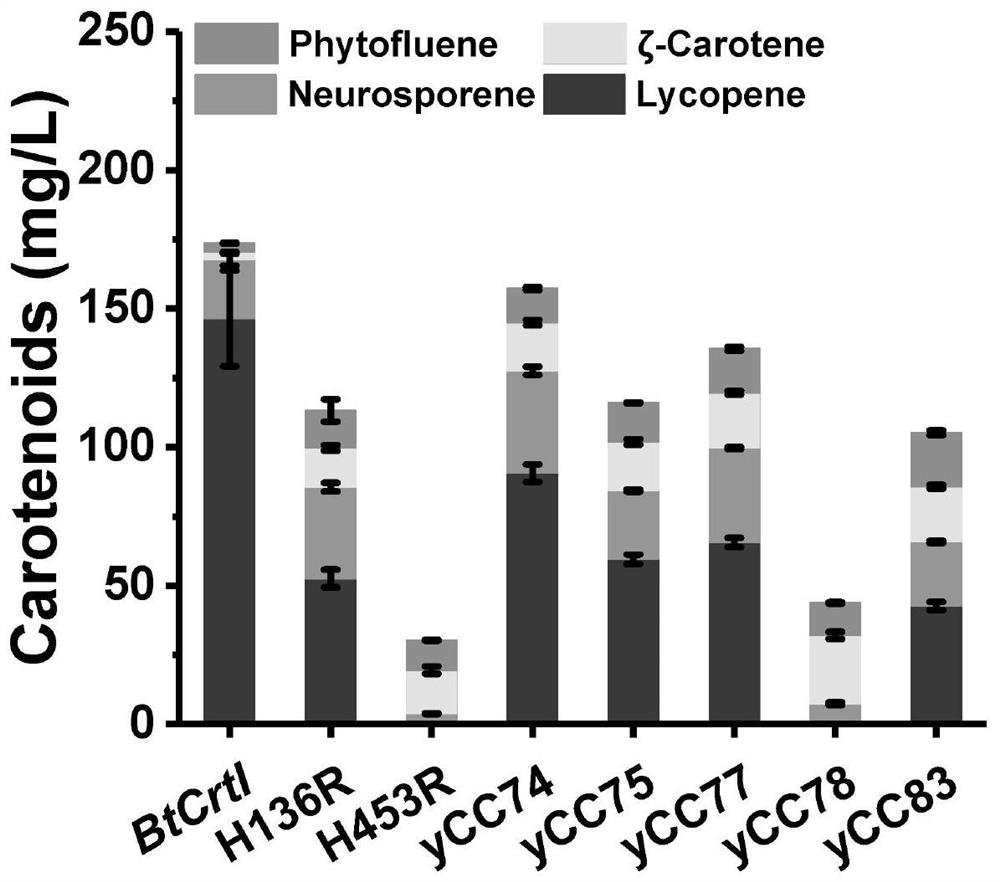
[0049] The second round of error-prone PCR did not screen out mutants with better performance. However, in the single-point verification test, it was found that the mutation points T545A, A289V, A355V, N148D, A289V&A355V can significantly improve the catalytic activity of CrtI, and the yield of lycopene has also been greatly improved. possible. The strains and fermentation results constructed by the single-point verification test are shown in Table 4, Figure 4 and table 5:
[0050] Table 4
[0051] strain descr...
Embodiment 3
[0057] Example 3 Exploring the Structural Features of Regulating CrtI Function
[0058] In order to explore the evolution rules of phytoene dehydrogenases from different sources at the H136 and H453 sites. We performed phylogenetic tree analysis and sequence alignment of phytoene dehydrogenases from bacteria, fungi, cyanobacteria, and plants. We found that phytoene dehydrogenases in RsCrtI, RcCrtI, and RaCrtI can catalyze a three-step dehydrogenation reaction, and the amino acid corresponding to the H136 position of BtCrtI is arginine; phytoene in cyanobacteria and plants The dehydrogenase can catalyze the two-step dehydrogenation reaction, and the amino acid corresponding to the H453 site of BtCrtI is also arginine, which is consistent with our experimental results, so we speculate that the two sites H136 and H453 are phyto Key structural sites of erythrin dehydrogenases that cause functional differentiation in natural evolution.
[0059] Then we analyzed the structural fea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com