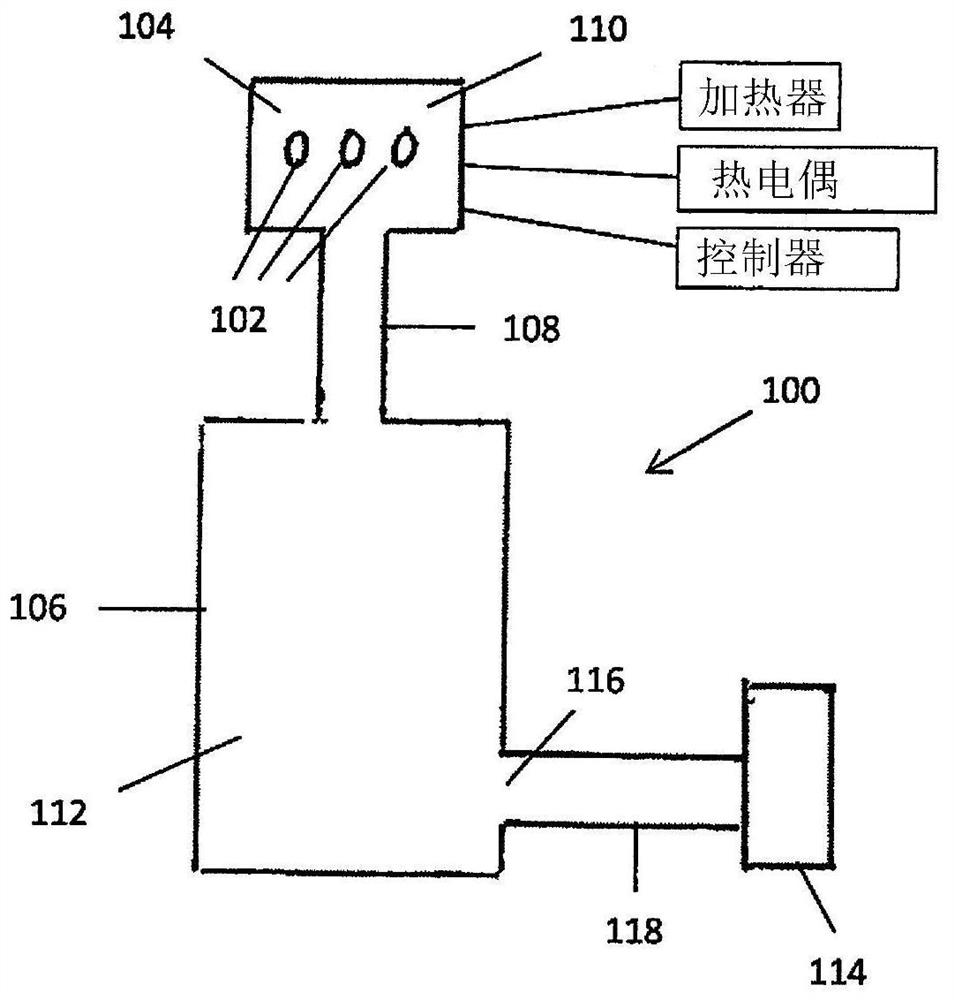
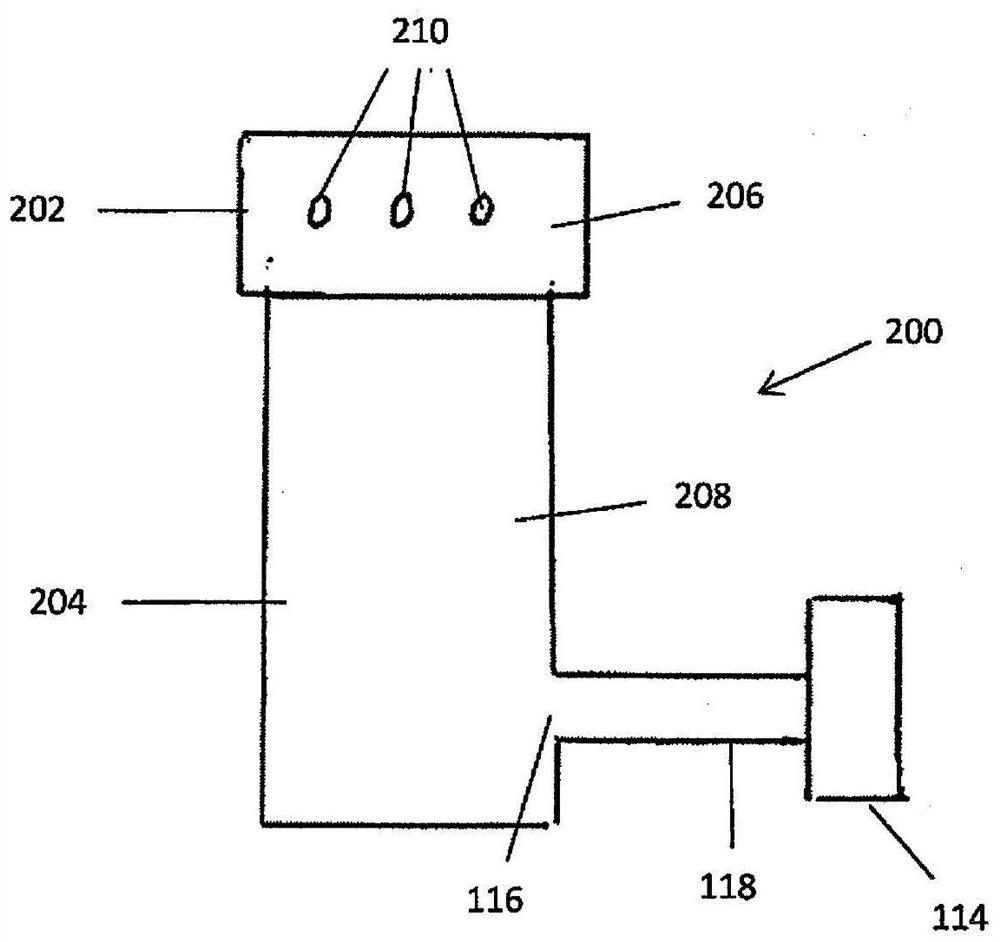
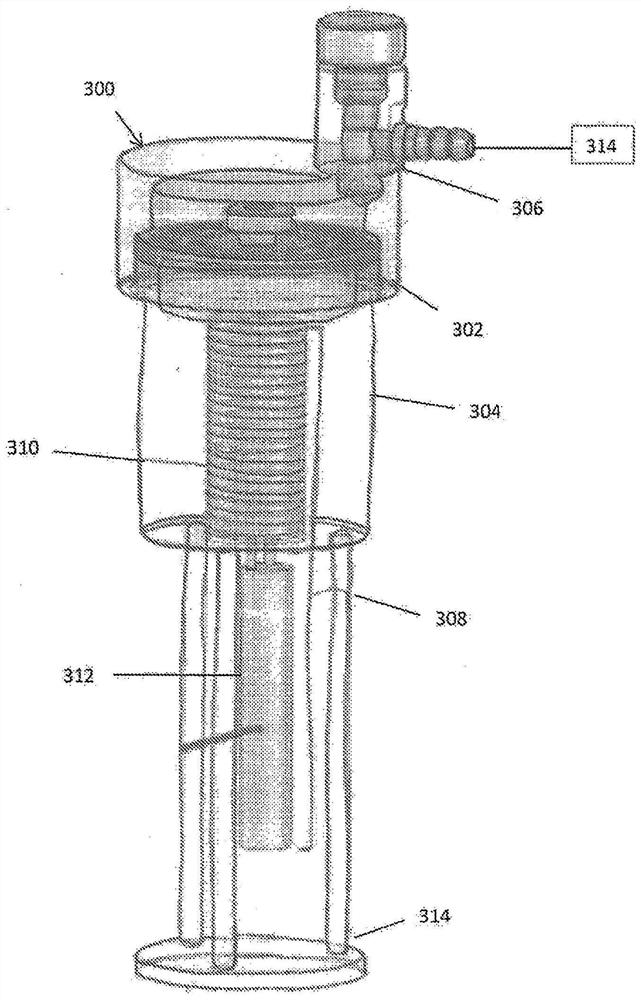
Device and method for freeze drying biological samples
A biological sample, drying technology, applied in the direction of drying solid materials, drying gas layout, preservation of human or animal bodies, etc., can solve the problems of irreversible loss of storage tank failure samples, expensive, troublesome transportation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0150] sperm collection
[0151] Sperm samples were collected from n=3 rams of the Sarda breed and pooled for analysis as a single sample. Concentration and motility were assessed using CASA (Ivos, Hamilton Thorne, Biosciences). Only sperm exhibiting motility of 85% or higher were considered for this experiment. Sperm samples were diluted to a concentration of 50 million in Tris medium supplemented with LyoA solution containing 0.25M trehalose and 0.4M sorbitol or Lyo B solution containing 0.16M trehalose and 0.26M sorbitol and 20% egg yolk sperm / ml. Sperm were then cooled at 1°C / min to 4°C before motility was reassessed using CASA.
[0152] freezing
[0153] In this experiment, freezing was accomplished by pipetting 10 [mu]l of sperm droplets onto coverslips pre-cooled to various temperatures (-10, -25 or -35[deg.] C.) for 1 hour. The coverslip was then removed and warmed by placing it on a warm plate (38°C).
[0154] Freeze drying
[0155] We used a new device (ca...
Embodiment 2
[0193] Name: Freeze-dried human spermatozoa show high DNA integrity after UV irradiation compared with frozen spermatozoa.
[0194] Research question: DNA integrity of a) frozen human sperm compared to b) frozen / dried (lyophilized) human sperm after UV irradiation.
[0195] Short answer: Freeze-dried human sperm maintains high DNA integrity compared to frozen sperm.
[0196] What is known: It was recently shown that mouse sperm that had been stored in a desiccated state on a space station for 9 months and exposed to cosmic radiation showed only minor DNA damage, which was repaired by oocytes and produced normal offspring.
[0197] Study Design, Size, Duration: Human spermatozoa were collected and frozen and freeze-dried. DNA integrity was measured using Hallosperm for: 1. Fresh control, 2. Freeze dried and rehydrated, 3. Freeze dried irradiated and rehydrated, 4. Freeze irradiated and thawed.
[0198] Participants / Materials, Settings, Methods:
[0199]Fresh human sperm sa...
Embodiment 3
[0205] Embodiment 3 (use ovarian tissue)
[0206] Mouse ovaries were dissected and cut into 1×10×5 mm sections. Ovarian sections were exposed to Lyo solution containing 10% DMSO, 10% HAS in PBS. After exposure to the LYO solution, the sections were placed inside straws with special pods (also called capsules) as described in PCT WO / 2017 / 064715A1 and cooled at a rate of 1 °C / min using a Darya apparatus.
[0207] Figure 12 and 13A The drying step is shown in . Place the cells on the surface or in the vial on the shelf. The vacuum monitor indicated a pressure of 10 mTorr and the condenser was set at a temperature of -115°C. Drying at relatively high negative temperatures, ie primary drying, is carried out by keeping the shelf temperature slightly below the Tg' of -30°C. Secondary drying with Darya was done by raising the shelf temperature by 10°C every hour until the desired storage temperature was reached - which could be LN to RT. At the conclusion of the primary and / or...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com