A Schiff base-organic zinc composition, its preparation method and its application in ring-opening polymerization
A ring-opening polymerization, organozinc technology, applied in the field of organic synthesis, can solve problems such as affecting product performance and limiting the application prospects of polymerized products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The present invention also provides a kind of preparation method of Schiff base-organic zinc composition, comprises the following steps:
[0037] Mixing the Schiff base compound with the structure of formula I and the organozinc compound with the structure of formula II, and activating at -20°C to 90°C for 0.5 to 12 hours to obtain the Schiff base-organozinc compound;
[0038] R 1 -Zn-R 2 Formula II;
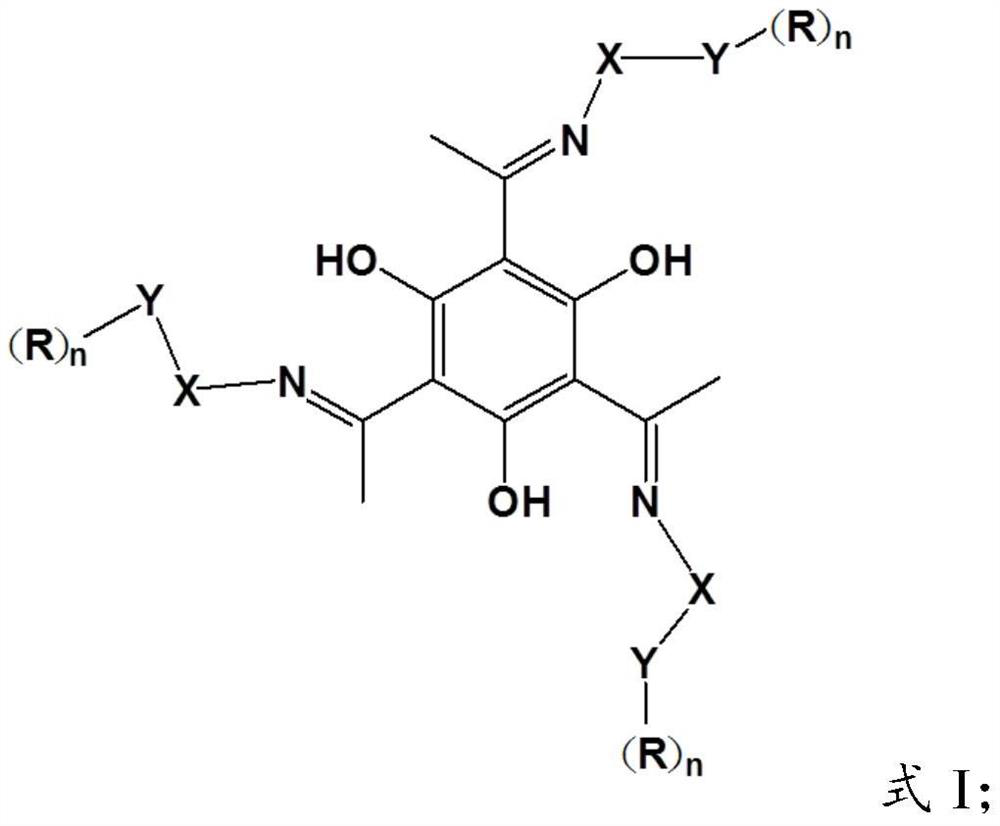
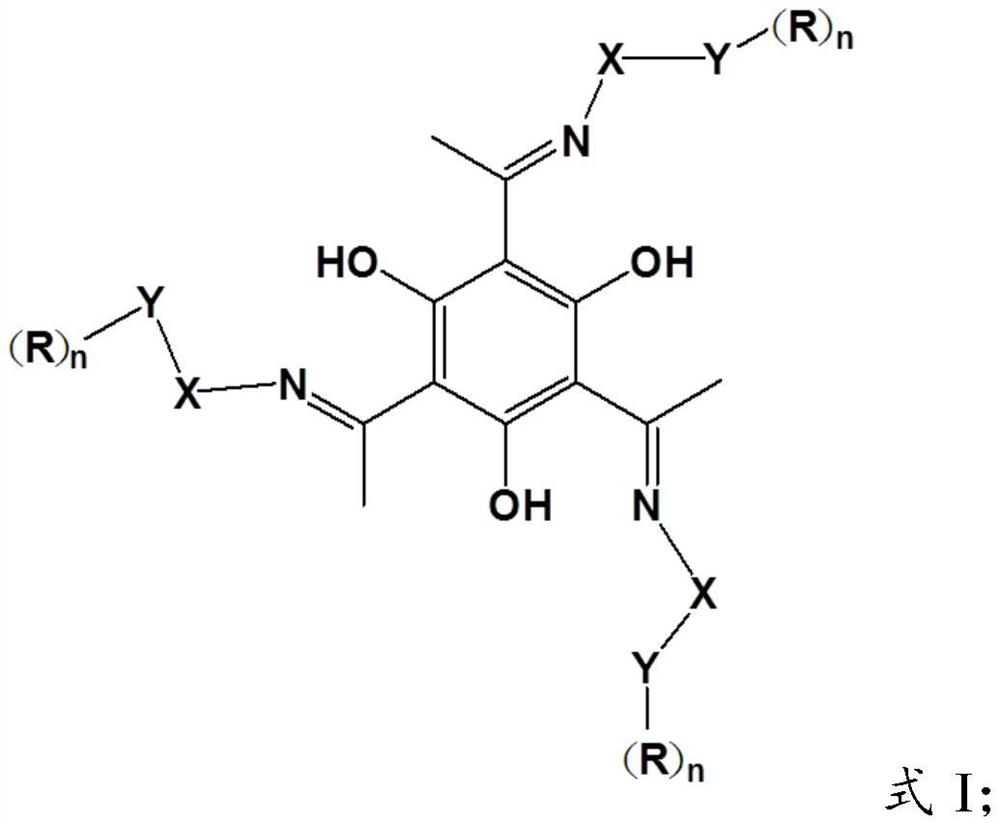
[0039] In formula I, R is selected from H, substituted or unsubstituted straight chain alkyl, or substituted or unsubstituted branched chain alkyl; n=1 or n=2; X is -(CH 2 )- m , 2≤m≤6; when n=1, Y is O, when n=2, Y is N;
[0040] In formula II, R 1 and R 2 are independently selected from substituted or unsubstituted straight-chain alkyl groups, or substituted or unsubstituted branched-chain alkyl groups.
[0041]In the present invention, the Schiff base compound has the same structure as the above-mentioned Schiff base compound having the structure of formula I,...
Embodiment 1
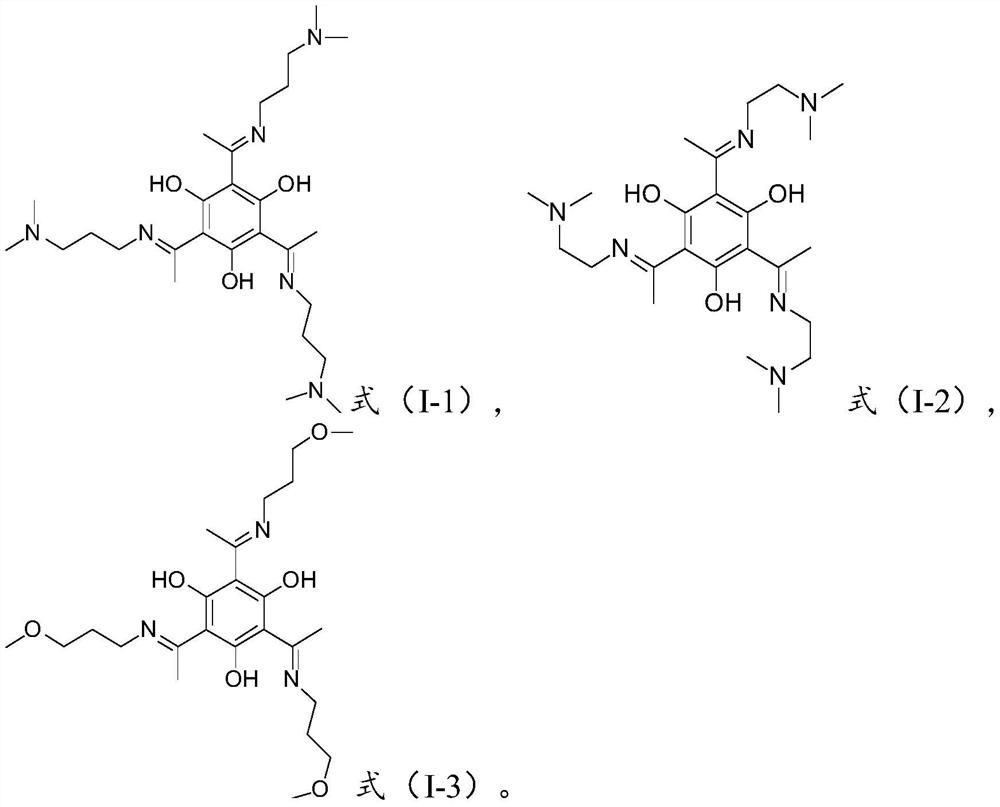
[0061] Weigh 1.0 g of the salicylaldehyde compound with the structure shown in formula (3) and dissolve it in 50 mL of absolute ethanol, add 2.2 g of N,N-dimethyl-1,3-propanediamine to it, and heat to reflux for 24 hours to react Most of the solvent of the reaction solution is removed by rotary evaporation, and the obtained reaction product is purified by column chromatography, and the Schiff base compound of the structure shown in formula (5) is obtained after separation and treatment.
[0062]
[0063] The resulting Schiff base was subjected to NMR testing, and the 1H NMR analysis results showed: 1H NMR: δ=3.41(m, NCH2CH2CH2N(CH3)2 2H), 2.55(s, ArCCH3 3H), 2.36(m, NCH2CH2CH2N(CH3) 2 2H), 2.22(s, N(CH3)2 6H), 1.81(m, NCH2CH2CH2N(CH3)2 2H).13C NMR: δ=185.44(ArCCH3), all benzenering: 170.34, 105.83; 56.42(NCH2CH2CH2N(CH3 )2), 45.17(NCH2CH2CH2N(CH3)2), 40.87(N(CH3)2, 27.44(NCH2CH2CH2N(CH3)2), 18.06(ArCCH3). NMR test results show that the Schiff base obtained in this embodimen...
Embodiment 2
[0065] Weigh 0.4 g of the salicylaldehyde compound having the structure shown in formula (3) and dissolve it in 50 mL of absolute ethanol, add 0.7 g of N,N-dimethylethylenediamine thereto, and heat to reflux for 24 hours to react; Most of the solvent in the solution, the obtained reaction product is purified by column chromatography, and the Schiff base compound with the structure shown in formula (6) is obtained after separation and treatment.
[0066]
[0067] The obtained Schiff base is carried out nuclear magnetic resonance test, 1 H NMR analysis results show: 1 H NMR: δ=3.44(m, NCH 2 CH 2 N(CH 3 ) 2 2H),2.57(m,NCH 2 CH 2 N(CH 3 ) 2 2H),2.54(s,ArCCH 3 3H),2.27(s,N(CH 3 ) 2 6H). 13 C NMR: δ=185.58 (ArCCH 3 ), all gasoline ring: 170.10, 106.51; 58.17 (NCH 2 CH 2 N(CH 3 ) 2 ),45.36(NCH 2 CH 2 N(CH 3 ) 2 ),41.31(N(CH 3 ) 2 ,18.25 (ArCCH 3 ). NMR test results show that the Schiff base obtained in the present embodiment has a structure shown in form...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com