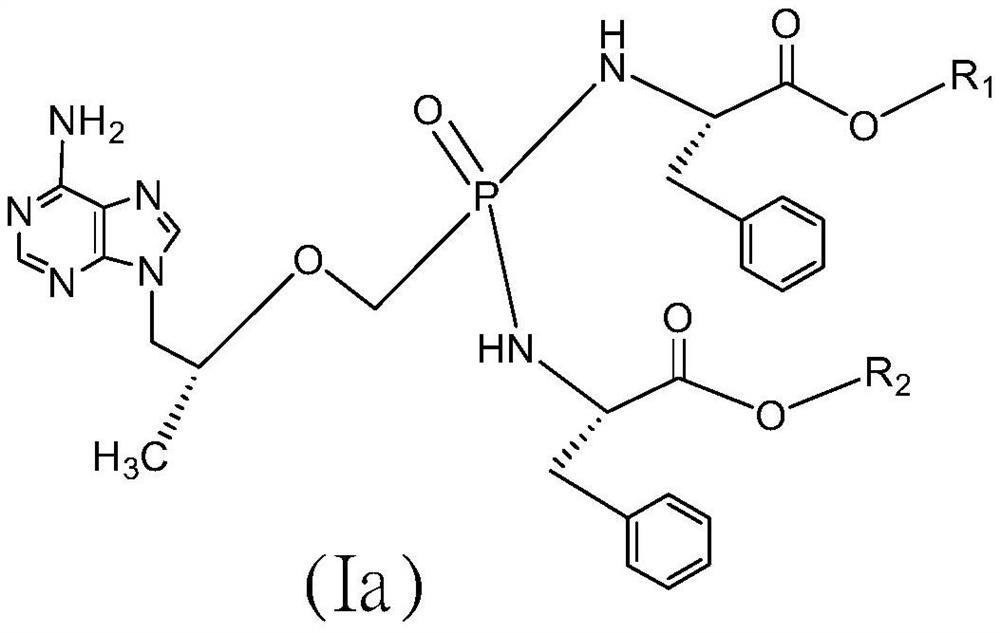
Tenofovir bisphenylpropionate phosphoramidate compound as well as pharmaceutical composition and application thereof
A technology of phosphoramidate and bisphenylpropionate, applied in the field of medicine, can solve the problems of bone and kidney side effects, certain drug resistance, small side effects, etc., and achieve a large biological activity selection coefficient, low toxicity, and high bioavailability. degree of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
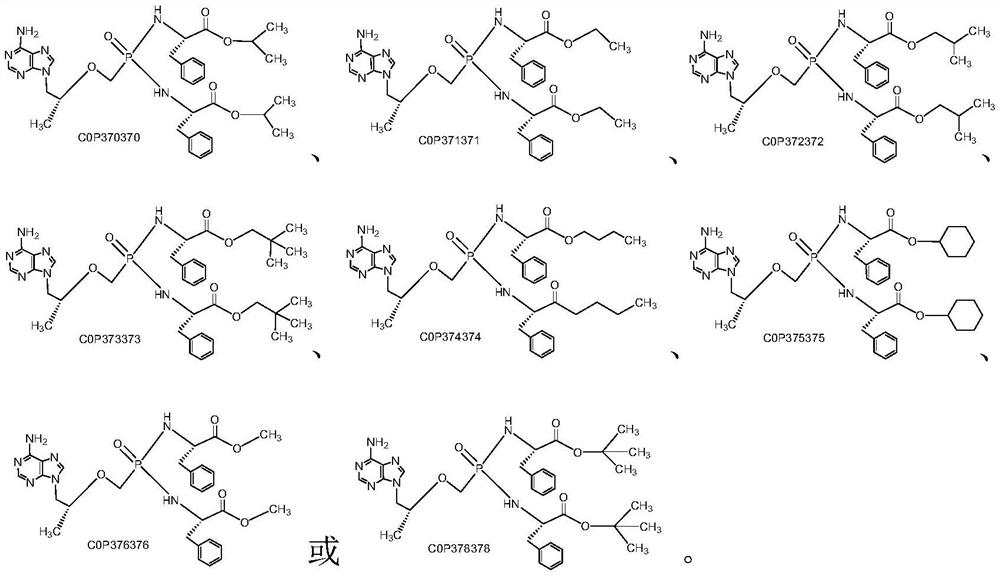
[0032] 9-[(R)-2-[[Di[[(S)-(1-n-butoxycarbonyl-2-phenyl)ethyl]amino]phosphinyl]methoxy]propyl]adenine Preparation of (COP374374).
[0033] Take commercially available tenofovir (COP00) 7.2g (25mmol), add in the 250ml flask, then add thionyl chloride 50g, heat up to 55°C, after 10 minutes, continue to heat up to 70°C and stir for 1 hour , distilled thionyl chloride under reduced pressure, added 100ml of dry acetonitrile after the distillation was completed and refluxed at 85°C for 10min, evaporated the acetonitrile to dryness under reduced pressure, cooled down to room temperature, then added 50ml of dry dichloromethane, and continued to stir for 0.5h Add 19.4g (75mmol, 3eq) of L-phenylalanine n-butyl hydrochloride (AH374), stir for another 10min, then add about 20ml of triethylamine dropwise, after the dropwise addition is complete, continue stirring for 0.5h.
[0034] After suction filtration, the filtrate was evaporated to dryness, and then 100ml of ethyl acetate was added. ...
Embodiment 2
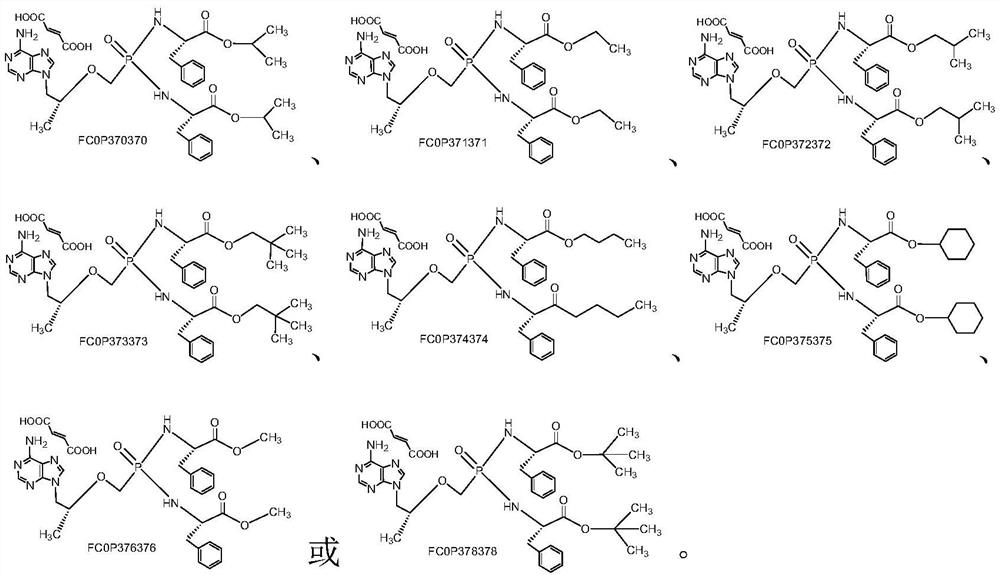
[0038] 9-[(R)-2-[[Di[[(S)-(1-n-butoxycarbonyl-2-phenyl)ethyl]amino]phosphinyl]methoxy]propyl]adenine Preparation of Fumarate (FCOP374374).
[0039]
[0040] An equivalent amount of 9-[(R)-2-[[two[[(S)-(1-n-butoxycarbonyl-2-phenyl)ethyl]amino]
[0041]Phosphinyl] methoxyl group] propyl group] adenine (COP374374) and fumaric acid were dissolved in hot acetonitrile, stirred at reflux for 2 hours, cooled and crystallized at room temperature, filtered out the precipitated solid and washed with acetonitrile to obtain a white solid: 9-[(R)-2-[[Di[[(S)-(1-n-butoxycarbonyl-2-phenyl)ethyl]amino]phosphinyl]methoxy]propyl]adenine Fumarate (FCOP374374).
Embodiment 3
[0043] In Vitro Determination of Anti-HBV Viral Activity of Compounds of the Present Invention
[0044] 1. In vitro cell model: HepG 2.2.15 cells
[0045] 2. Dot blot method to determine the anti-hepatitis B virus activity of the compound
[0046] 2.1 HepG 2.2.15 cells (4×10 4 Cells / well) into 96-well plates, cultured overnight at 37°C with 5%.
[0047] 2.2 On the second day, dilute the compound and add different concentrations of the compound to the culture wells. The final concentration of DMSO in the culture medium was 1%. 1 μM entecavir (ETV) was used as 100% Inhibition control; 1% DMSO was used as 0% Inhibition control.
[0048] 2.3 On the fifth day, replace with fresh culture medium containing the compound.
[0049] 2.4 On the eighth day and the ninth day, the culture medium in the culture well was removed, and the cells were harvested for dot hybridization.
[0050] 3. Results: See Table 1.
[0051] Table 1: Cytotoxicity of compounds, extracellular anti-HBV activ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com