Covalent organic framework material as well as preparation method and application thereof
A covalent organic framework and reaction technology, applied in organic chemistry, chemical instruments and methods, organic compound/hydride/coordination complex catalysts, etc., can solve poor stability, difficult preparation of COF materials, low reaction catalyst efficiency, etc. problem, to achieve the effect of mild reaction conditions, less catalyst dosage, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
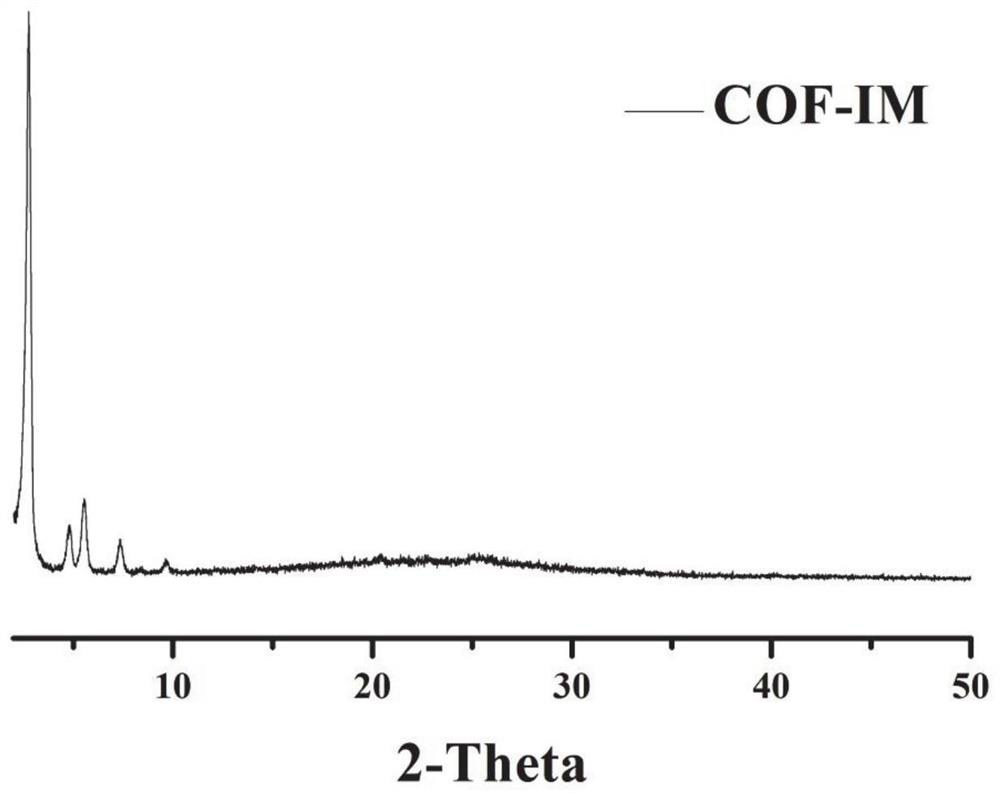
Image
Examples
preparation example Construction
[0055] Further, the preparation method includes the following steps: using o-dichlorobenzene and n-butanol as solvents in acid, BF 3 ·OEt 2 and 2,3-dichloro-5,6-dichloro-5,6-dicyano-1,4-benzoquinone (DQO), three components of L1, L2 and L3 occur under solvothermal conditions Povarov reacted, cooled to room temperature, centrifuged, washed with tetrahydrofuran, and dried in vacuum to obtain the product.
[0056] Preferably, the solvothermal condition is 90-120°C,
[0057] Further preferably, the solvothermal temperature is 120°C,
[0058] Preferably, the reaction time of the Povarov reaction is 3 to 7 days,
[0059] Preferably, the reaction time of the Povarov reaction is 3 days;
[0060] Preferably, the acid is an organic acid,
[0061] Further preferably, the acid is glacial acetic acid, benzoic acid, trifluoroacetic acid,
[0062] Further preferably, the acid is glacial acetic acid,
[0063] Preferably, the molar ratio of L1, L2 and L3 is 1-3:2-5:5-10,
[0064] Furth...
Embodiment 1
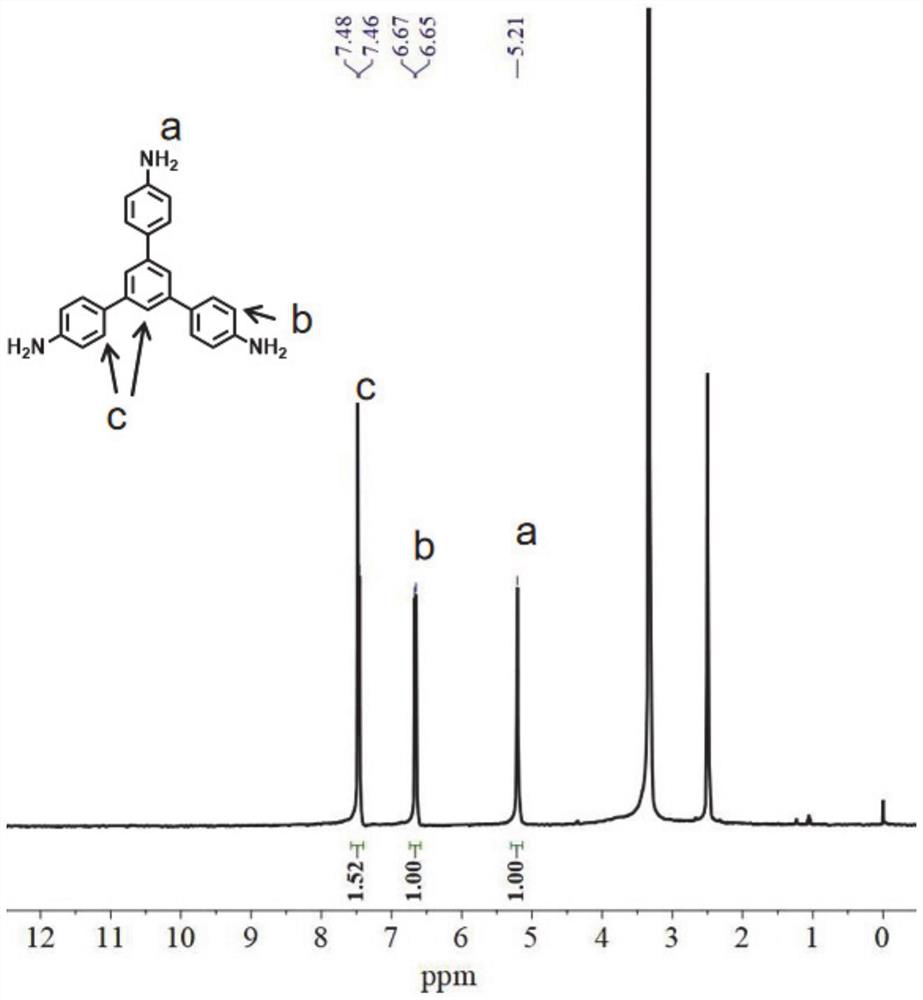
[0108] Embodiment 1: the preparation of organic ligand L1
[0109] (1) 4-nitroacetophenone (25g), toluene (100mL) and CF 3 SO 3H (1 mL) was added to a flask equipped with a water separator and a cooling condenser. The mixture was refluxed for 48 hours, during which time the water formed was eliminated as a toluene azeotrope. After cooling to room temperature, the mixture was filtered to obtain a black solid product. Wash with N,N-dimethylformamide (DMF) at reflux and filter. This was carried out two more times, and after drying, a light yellow solid was obtained, which was the intermediate (1,3,5-tri(4-nitrobenzene)benzene).
[0110] (2) A suspension of 1,3,5-tris(4-nitrophenyl)benzene (10 g, 22.7 mmol) and Pd / C (10 wt%, 2.0 g) in ethanol (200 mL) was heated to reflux. Hydrazine hydrate (30 mL) was added dropwise, and the mixture was refluxed overnight. The hot solution was filtered through celite and left undisturbed to completely crystallize the product. The solid was...
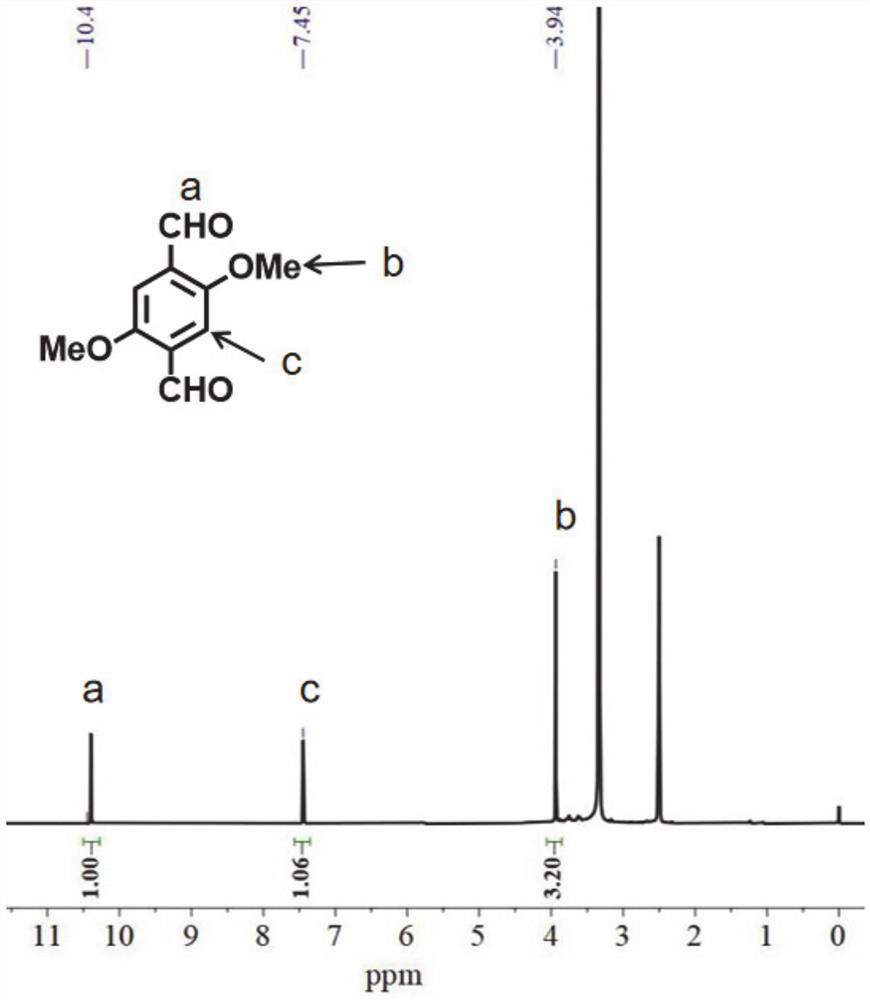
Embodiment 2
[0111] Embodiment 2: the preparation of organic ligand L2
[0112] (1) Add formaldehyde solution (37wt%, 10mL) and paraformaldehyde (6.0 g, 200 mmol). The resulting mixture was heated to 90°C, then concentrated hydrochloric acid (20 mL) was added dropwise. After heating for an additional 1 h, HCl (37 wt%, 60 mL) was introduced and the mixture was cooled to room temperature to give a white precipitate which was collected by filtration, rinsed with water, and dried under vacuum. The crude product was recrystallized from acetone to obtain a white powdery product, which was the intermediate 1,4-bis(chloromethyl)-2,5-dimethoxybenzene.
[0113] (2) Intermediate 1,4-bis(chloromethyl)-2,5-dimethoxybenzene (10.0g, 42.5mmol) and hexamethylenetetramine (12.0g, 85mmol) were stored in chloroform ( 100 mL) was stirred at 90 °C for 24 h. After cooling to room temperature, the pale yellow precipitate was collected by filtration and washed with CHCl 3 Wash, dry and dissolve in water. The...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com