Electrochemical synthesis method of 3-arylseleno quinolinone compound
The technology of an arylselenoquinolinone and a synthesis method, which is applied in a method field, can solve the problems of increasing the difficulty of product separation and purification, increasing the reaction cost and the like, and achieves the advantages of large-scale production, reduction of recovery steps and wide selectivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~5
[0058] The following examples 1 to 5 all react according to the following reaction equation, mainly to investigate the yield situation of different substrates reacting under optimal conditions:
[0059]
[0060] The specific operation steps are: in a 25mL three-neck round bottom flask, add quinolinone (6mmol), diaryl diselenide (3mmol), potassium iodide (0.9mmol), DMSO (12mL) in sequence, two 15mm×15mm×3mm The RVC electrodes serve as anode and cathode, respectively. The resulting mixture was stirred and reacted under a 12mA direct current at room temperature. The progress of the reaction was followed by thin-layer chromatography, and the reaction time was 10 hours. After the reaction was completed, 12ml of water was added and the product was precipitated, filtered and dried to obtain the pure product.
Embodiment 1
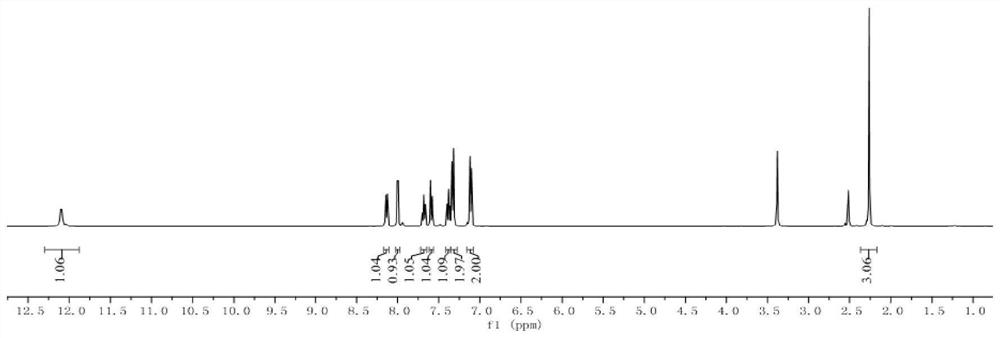
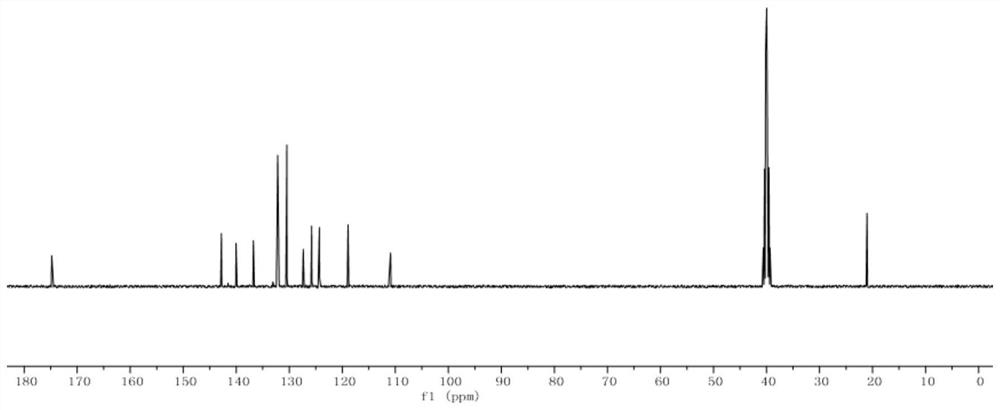
[0062] Compound 1,3-(p-tolylselanyl)quinolin-4(1H)-one, yield 98%.
[0063]
[0064] 1 H NMR (400MHz, DMSO-d 6 )δ12.09(s,1H),8.13(d,J=7.4Hz,1H),8.00(d,J=7.2Hz,1H),7.67(t,J=7.2Hz,1H),7.59(d, J=8.2Hz, 1H), 7.36(t, J=8.2Hz, 1H), 7.33(d, J=8.2Hz, 2H), 7.12(d, J=8.2Hz, 2H), 2.27(s, 3H) ;
[0065] 13 C NMR (100MHz, DMSO-d 6 )δ174.8, 142.8, 140.1, 136.8, 132.4, 132.2, 130.5, 127.4, 125.8, 124.5, 124.3, 118.9, 110.9, 21.1.
Embodiment 2
[0067] Compound 2, 3-(phenylselanyl)quinolin-4(1H)-one, yield 95%.
[0068]
[0069] 1 H NMR (400MHz, DMSO-d 6 )δ12.20(s,1H),8.20-8.14(m,2H),7.72-7.69(m,1H),7.68-7.61(m,1H),7.41(t,J=7.2Hz,3H),7.29 -7.20(m,3H);
[0070] 13 C NMR (100MHz, DMSO-d 6 )δ175.0, 144.3, 140.2, 132.5, 131.9 131.0, 129.7, 126.9, 125.8, 124.2, 124.4, 119.0, 109.7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com