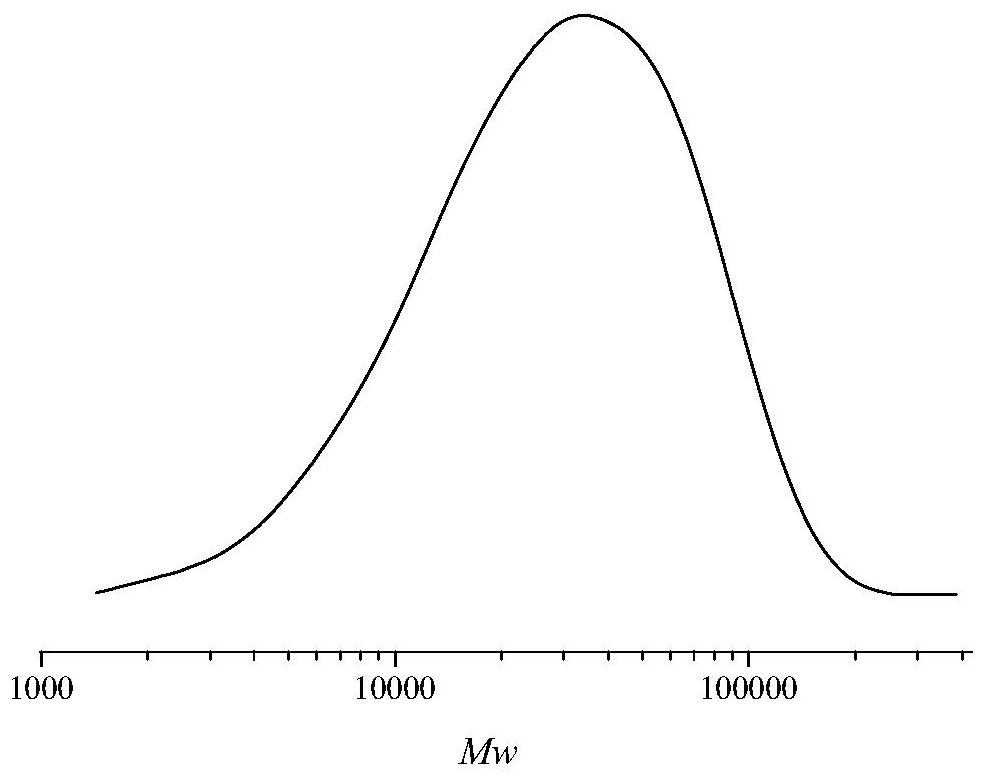
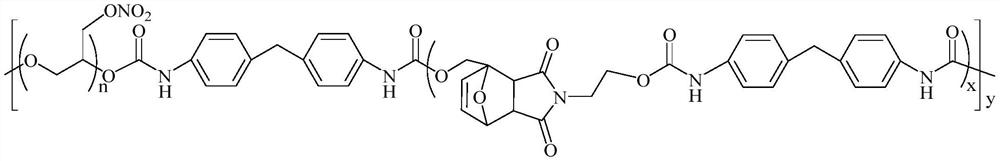
Polyglycidyl ether nitrate (PGN)-based thermoplastic polyurethane elastomer containing Diels-Alder bonds and one-pot preparation method of PGN-based thermoplastic polyurethane elastomer
A thermoplastic polyurethane and elastomer technology, used in offensive equipment, non-explosive/non-thermal agent components, non-explosive fillers/gelling agents/thickeners, etc. To achieve multiple repairs and other problems, to achieve the effect of high energy characteristics and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] This embodiment provides a PGN-based thermoplastic polyurethane elastomer containing a Diels-Alder bond, which is prepared according to the following one-pot method:
[0030] In a 100mL three-neck round-bottom flask equipped with mechanical stirring, a thermometer and a reflux device, add dry PGN (4g, 2mmol) with a molecular weight of 2000Da, MDI (3g, 12mmol), 1.2mg of dibutyltin dilaurate and 15mL of anhydrous Chloroform forms a mixed solution. Under a nitrogen atmosphere, the mixed solution was placed in a water bath at 80° C., and mechanically stirred for 2 hours to obtain an isocyanate-terminated polyurethane prepolymer solution. Cool the mixed solution to 60°C, add 1-hydroxymethyl-10-oxatricyclo[5.2.1.02,6]dec-8-ene-3,5-dione-2-aminoethanol (2.4g, 10mmol ), continue to react in a water bath at 80° C. for 4 h under a nitrogen atmosphere. After the reaction was completed, the reaction system was concentrated under reduced pressure, and the yield of the light yellow...
Embodiment 2
[0035] This embodiment provides a PGN-based thermoplastic polyurethane elastomer containing a Diels-Alder bond, which is prepared according to the following one-pot method:
[0036] In a 100mL three-neck round bottom bottle equipped with mechanical stirring, thermometer and reflux device, add dry PGN (6g, 2mmol) with a molecular weight of 3000Da, MDI (2g, 8mmol), 1.5mg dibutyltin dilaurate and 14mL anhydrous Dichloroethane forms a mixed solution. Under a nitrogen atmosphere, the mixed solution was placed in a water bath at 85° C., and mechanically stirred for 3 h to obtain an isocyanate group-terminated polyurethane prepolymer solution. Cool the mixed solution to 65°C, add 1-hydroxymethyl-10-oxatricyclo[5.2.1.02,6]dec-8-ene-3,5-dione-2-aminoethanol (1.44g, 6mmol ), continue to react in a water bath at 85° C. for 3 h under a nitrogen atmosphere. After the reaction is finished, the reaction system is concentrated under reduced pressure, and the obtained light yellow elastomer ...
Embodiment 3
[0038] This embodiment provides a PGN-based thermoplastic polyurethane elastomer containing a Diels-Alder bond, which is prepared according to the following one-pot method:
[0039] In a 100mL three-neck round-bottom flask equipped with mechanical stirring, a thermometer and a reflux device, add dry PGN (8g, 2mmol) with a molecular weight of 4000Da, MDI (1.5g, 6mmol), 1.8mg dibutyltin dilaurate and 16mL no Water and toluene form a mixed solution. Under a nitrogen atmosphere, the mixed solution was placed in a 90° C. water bath, and mechanically stirred for 4 hours to obtain an isocyanate-terminated polyurethane prepolymer solution. Cool the mixed solution to 60°C, add 1-hydroxymethyl-10-oxatricyclo[5.2.1.02,6]dec-8-ene-3,5-dione-2-aminoethanol (0.96g, 4mmol ), and continued to react in a water bath at 90° C. for 5 h under a nitrogen atmosphere. After the reaction is finished, the reaction system is concentrated under reduced pressure, and the obtained light yellow elastomer ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com