Type-B avian infectious coryza subunit vaccine as well as preparation method and application thereof
A chicken infectious rhinitis and subunit vaccine technology, which is applied in the directions of botanical equipment and method, biochemical equipment and method, application, etc., can solve the problems of high culture cost, side reactions, focal necrotic spots, etc. Simple process, low cost, excellent immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
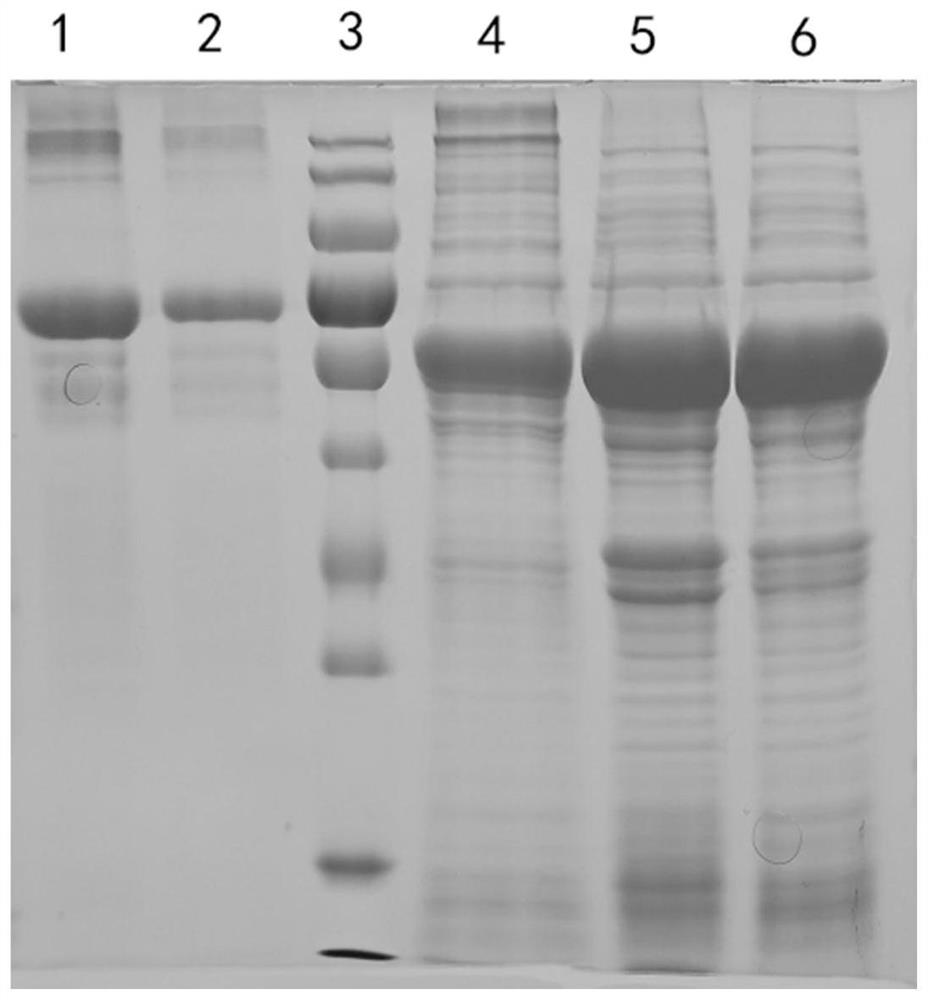
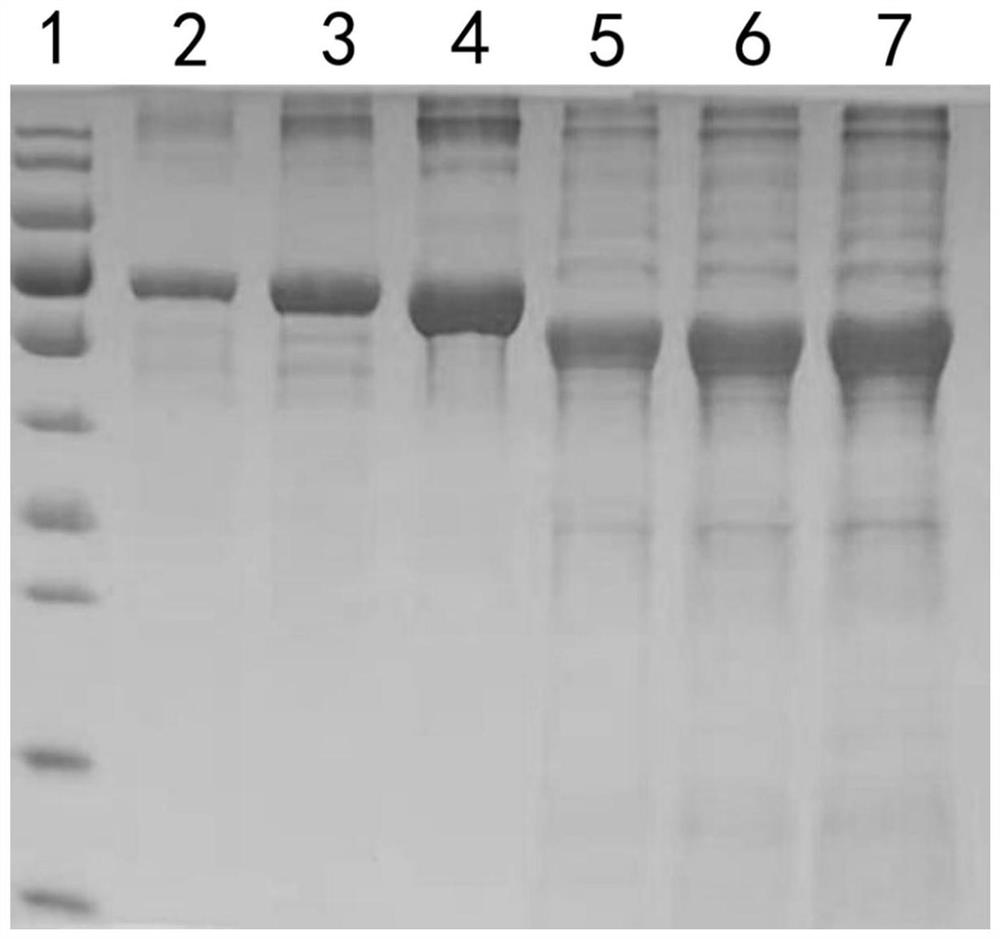
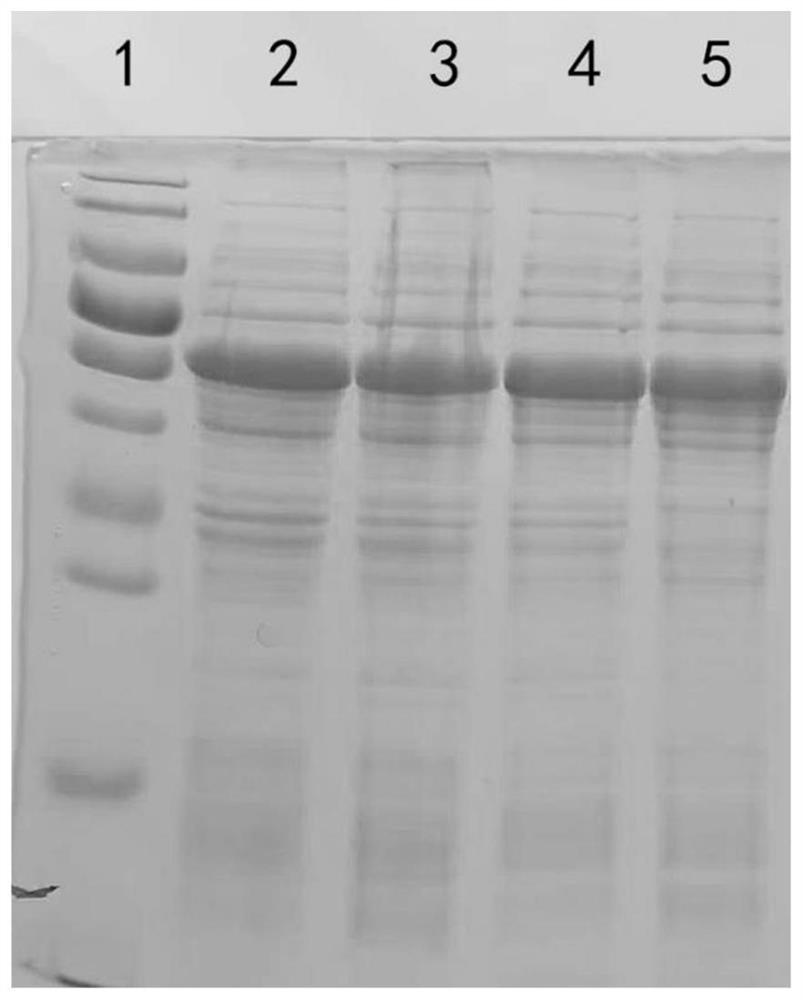
[0026] 1. Optimization of B-HMTp210-R2 nucleotide sequence
[0027] In order to improve the expression level, soluble expression level and stability of target protein, the present embodiment is to the nucleotide sequence (as shown in SEQ ID NO.3) and amino acid of B-type chicken infectious rhinitis outer membrane protein B-HMTp210-R2 The sequence has been optimized in many ways. After a large number of screening and comparative experiments, the present invention finally determines the optimization scheme of the B-HMTp210-R2 nucleotide sequence (the optimized nucleotide sequence is shown in SEQ ID NO.2):
[0028] 2. Soluble expression of B-HMTp210-R2 protein
[0029] The above-mentioned B-HMTp210-R2 gene sequence optimized by nucleotide sequence was synthesized, and B-HMTp210-R2 before and after nucleotide sequence optimization were respectively digested with Nde I / Xho I and cloned into the pET-30a vector, The obtained recombinant expression vectors were named pET30a-B-HMTp21...
Embodiment 2
[0031] The present embodiment provides a preparation process of type B chicken infectious rhinitis subunit vaccine, and the specific steps are as follows:
[0032] 1. Expression of B-HMTp210-R2 protein:
[0033] (1) Activation of genetically engineered bacteria, get 10 μL of engineered bacteria pET30a-B-H MTp210-R2-OPTI glycerolbacteria constructed in Example 2 and inoculate in 5ml of kana-resistant LB medium, culture overnight with shaking;
[0034] (2) Inoculate 200mL of Kanna-resistant culture medium at a ratio of 1:200, culture with shaking at 37°C for 4-5 hours, until the OD600 value reaches about 0.8;
[0035] (3) Add IPTG at a ratio of 1:2000, and induce at 16°C for 15 hours;
[0036] (4) collect thalline;
[0037] (5) Resuspend bacteria with 20mL special buffer;
[0038] (6) Ultrasonic disruption of bacteria;
[0039] (7) 12000rpm centrifugal 10min, centrifugal 2 times;
[0040] (8) Add 40% ammonium sulfate to precipitate the target protein, centrifuge at 12000rpm...
Embodiment 3
[0057] The present embodiment carries out efficacy analysis to the type B chicken infectious rhinitis subunit vaccine prepared in embodiment 2, specifically as follows:
[0058] (1) The safety test of the minimum age of use and a single-dose inoculation by different routes
[0059] Divide 70 7-day-old SPF chickens into 4 groups, with 20 chickens in the immunization group 1-3, 10 chickens in the control group in the 4th group, and 10 chickens in the control group in the 1-3 immunization group. Each group is further divided into two groups, with 10 SPF chickens in each group Chickens were inoculated with Type B chicken infectious rhinitis subunit inactivated vaccines (batches were 20190712, 20190805 and 20190820 respectively, the preparation method was the same as in Example 2) by different immunization routes (intramuscular injection or neck subcutaneous injection), and the dose was 0.5mL / bird; 10 SFP chickens in the control group were subcutaneously injected with sterilized ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com