Iron-niobium oxide as well as preparation method and application thereof
A technology of oxide and iron niobium, which is applied in the field of iron niobium oxide and its preparation, can solve the problems of poor catalytic effect and low space velocity of catalysts, and achieve the effect of improving catalytic performance and good catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
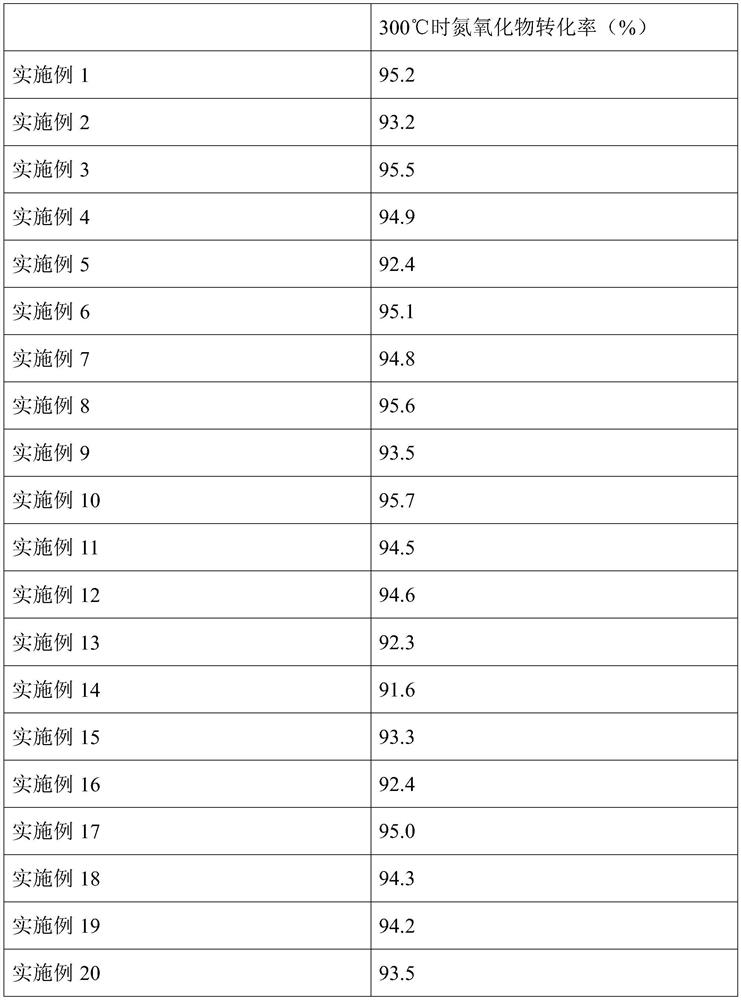
Embodiment 1
[0061] This embodiment provides a method for preparing iron niobium oxide, which specifically includes:
[0062] (I) take ferric nitrate nonahydrate and niobium oxalate by mass ratio 2:1; Configuration concentration is the CTAB aqueous solution of 30mmol / L, wherein, the mass ratio of CTAB in ferric nitrate nonahydrate and CTAB aqueous solution is 3.7:1, will weigh Add ferric nitrate nonahydrate and niobium oxalate into CTAB aqueous solution and mix and stir at 20°C for 1 hour to obtain a mixture. Add 25 wt% ammonia solution to the mixture to make the pH of the reaction system 11, stir and mix at 20°C for 3 hours to obtain a mixed solution ;
[0063] (II) The mixed solution is heated in a water bath at 90°C for 10 hours. After heating, suction filtration and washing are carried out to obtain a solid product. The solid product is dried at 100°C. After drying, it is calcined at 400°C for 5 hours. The rate is 2°C / min, and iron niobium oxide is obtained.
[0064] Adopt above-ment...
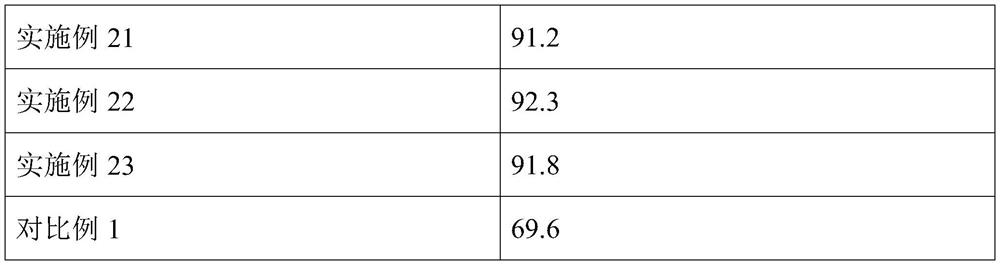
Embodiment 2
[0067] This embodiment provides a method for preparing iron niobium oxide, which specifically includes:
[0068] (I) take ferric nitrate nonahydrate and niobium oxalate by mass ratio 2:1; Configuration concentration is the CTAB aqueous solution of 5mmol / L, wherein, the mass ratio of CTAB in ferric nitrate nonahydrate and CTAB aqueous solution is 3.7:1, will weigh Add ferric nitrate nonahydrate and niobium oxalate into CTAB aqueous solution and mix and stir at 22°C for 0.5h to obtain a mixture, add 26wt% ammonia solution to the mixture to make the pH of the reaction system 11, stir and mix at 22°C for 1h to obtain a mixed solution ;
[0069] (II) The mixed solution is heated in a water bath at 60°C for 15 hours, and then filtered and washed with suction to obtain a solid product. The solid product is dried at 100°C, and after drying, it is calcined at 500°C for 5 hours. The rate is 1°C / min, and iron niobium oxide is obtained.
[0070] Adopt above-mentioned preparation method ...
Embodiment 3
[0073] This embodiment provides a method for preparing iron niobium oxide, which specifically includes:
[0074] (I) take ferric nitrate nonahydrate and niobium oxalate by mass ratio 2:1; Configuration concentration is the CTAB aqueous solution of 50mmol / L, wherein, the CTAB mass ratio in ferric nitrate nonahydrate and CTAB aqueous solution is 3.7:1, will weigh Add ferric nitrate nonahydrate and niobium oxalate into CTAB aqueous solution and mix and stir at 30°C for 3 hours to obtain a mixture, add 22wt% ammonia solution to the mixture to make the pH of the reaction system 11, stir and mix at 30°C for 5 hours to obtain a mixed solution;
[0075] (II) The mixed solution is heated in a water bath at 95°C for 5 hours, and then filtered and washed with suction to obtain a solid product. The solid product is dried at 120°C, and after drying, it is calcined at 400°C for 3 hours. The rate is 10°C / min to obtain iron niobium oxide.
[0076] Adopt above-mentioned preparation method to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com