Methylene-bridged nitrogen-rich diheterocyclic compound, derivative and preparation method thereof
A methylene bridge and compound technology is applied in the field of methylene bridged nitrogen-rich biheterocyclic compounds and their derivatives, and can solve the problems of difficulty in designing new energetic materials, achieve low density and prevent decomposition. , the effect of high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
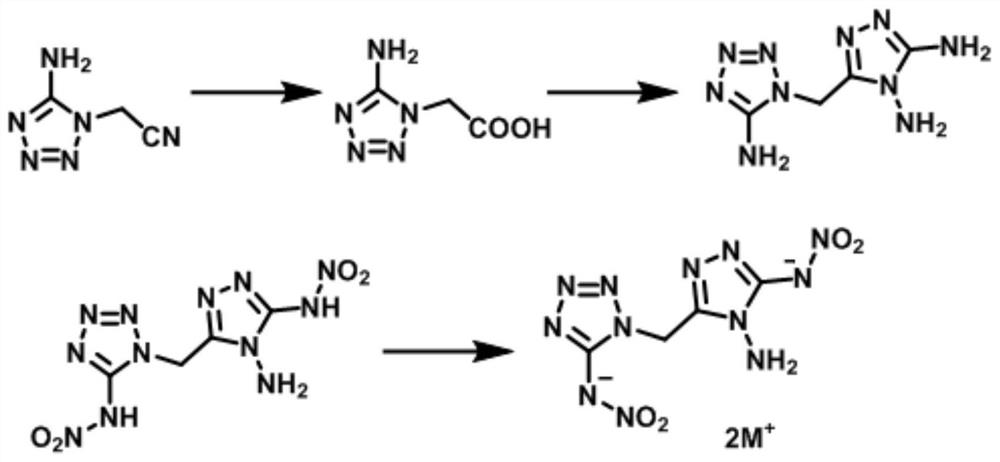
[0034] A method for preparing a methylene bridged nitrogen-rich biheterocyclic compound of the present invention comprises the following steps:
[0035] Step 1: Dissolve 2mmol of 1-acetonitrile 5-aminotetrazole in 20ml of 1M NaOH aqueous solution, reflux at 100-105°C for 1.5-2.5h to carry out the oxidation reaction, after returning to room temperature, add hydrochloric acid dropwise to adjust the pH =7, the acidification reaction was carried out to free the product, the product was precipitated, and the precipitate was filtered out. The precipitate was 1-acetoxy 5-aminotetrazole, accompanied by a small amount of impurities, but did not affect the subsequent reaction.
[0036]Step 2: Dissolve 0.214g of the precipitate obtained in step 1 in 10ml of polyphosphoric acid, stir at 40°C for 5 minutes to fully dissolve the precipitate, then add 2mmol of diaminoguanidine hydrochloride, and heat up to 110-130°C for addition After 12-14 hours of reaction, the desired substance was formed...
Embodiment 1
[0053] Step 1: Dissolve 2mmol of 1-acetonitrile 5-aminotetrazole in 20ml of 1 M NaOH aqueous solution, reflux at 100°C for 2h, after returning to room temperature, add hydrochloric acid dropwise to adjust the pH to 7, and filter out the precipitate after the reaction.
[0054] Step 2: Dissolve 0.214 g of the solid obtained in step 1 in 10 ml of polyphosphoric acid, stir at 40° C. for 5 minutes, add 2 mmol of solid diaminoguanidine hydrochloride, and heat up to 120° C. for 12 hours. After the reaction, return to room temperature, add 20 g of ice water at 2° C., adjust pH=8, and filter compound 1.
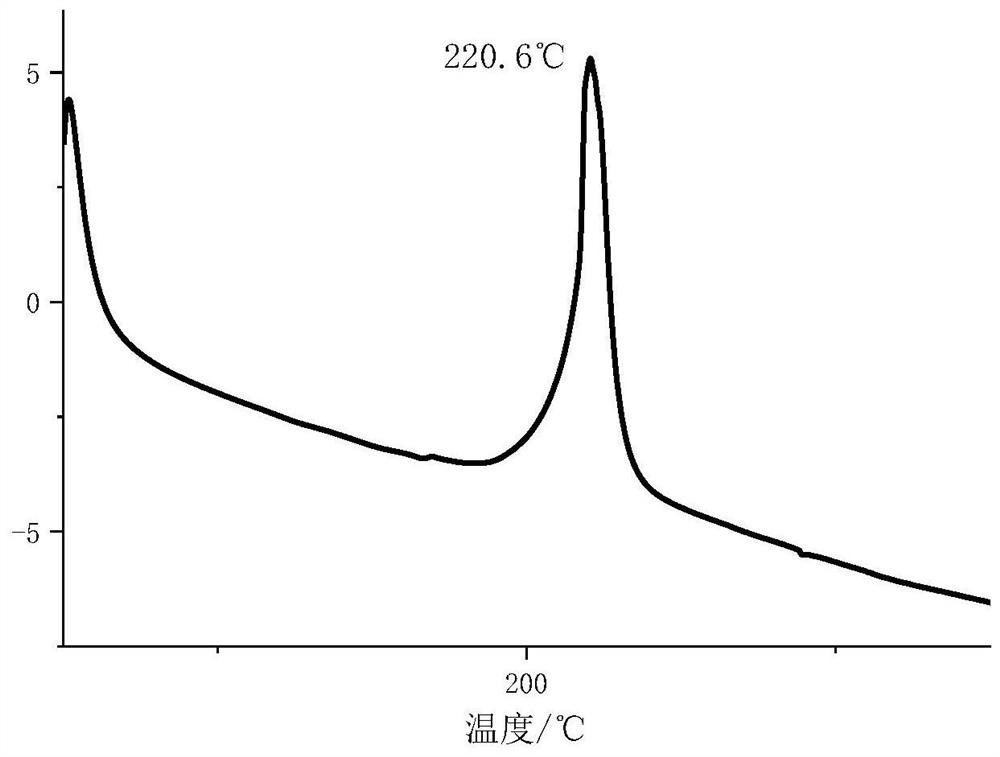
[0055] From Figure 4 It can be seen that compound 1 has a downward endothermic peak, indicating that it melts before being nitrated, that is, before being dissolved in fuming nitric acid, and then decomposes to carry out oxidation reaction. The heat of combustion at constant volume of compound 1 is 6000J / g, IS>40J, FS>360N, indicating low energy and high stability.
[0056] Table ...
Embodiment 2
[0064] Step 1: Dissolve 2mmol of 1-acetonitrile 5-aminotetrazole in 20ml of 1 M NaOH aqueous solution, reflux at 105°C for 2.5h, after returning to room temperature, add hydrochloric acid dropwise to adjust PH=7, filter out the precipitate after the reaction .
[0065] Step 2: Dissolve 0.214 g of the solid obtained in step 1 in 10 ml of polyphosphoric acid, stir at 40° C. for 5 minutes, add 2 mmol of solid diaminoguanidine hydrochloride, and heat up to 110° C. for 14 hours. After the reaction, return to room temperature, add 20 g of ice water at 0° C., adjust pH=9, and obtain compound 1 by filtration.
[0066] Step 3: Take 1ml of fuming nitric acid and stir at -5°C for 10min, take 0.1g of compound 1 and dissolve in fuming nitric acid at -5°C, slowly return to room temperature, and stir for 7h. After the reaction was completed, it was dropped on ice, and the precipitated white solid was filtered and dried to obtain dinitrate ammonium compound 2.
[0067] Step 4: 2 mmol of com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com