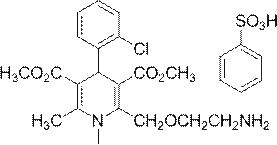
Amlodipine atorvastatin calcium liquid-solid compressed tablet and preparation method thereof
A technology of atorvastatin calcium and amlodipine, which is applied in the direction of pharmaceutical formulas, medical preparations with no active ingredients, and medical preparations containing active ingredients, etc., which can solve the problem that the stability of atorvastatin calcium cannot be guaranteed and affects Amlodipine stability, lack of alkaline stabilizers and other issues, achieve the effect of low cost, avoid instability, and ensure stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
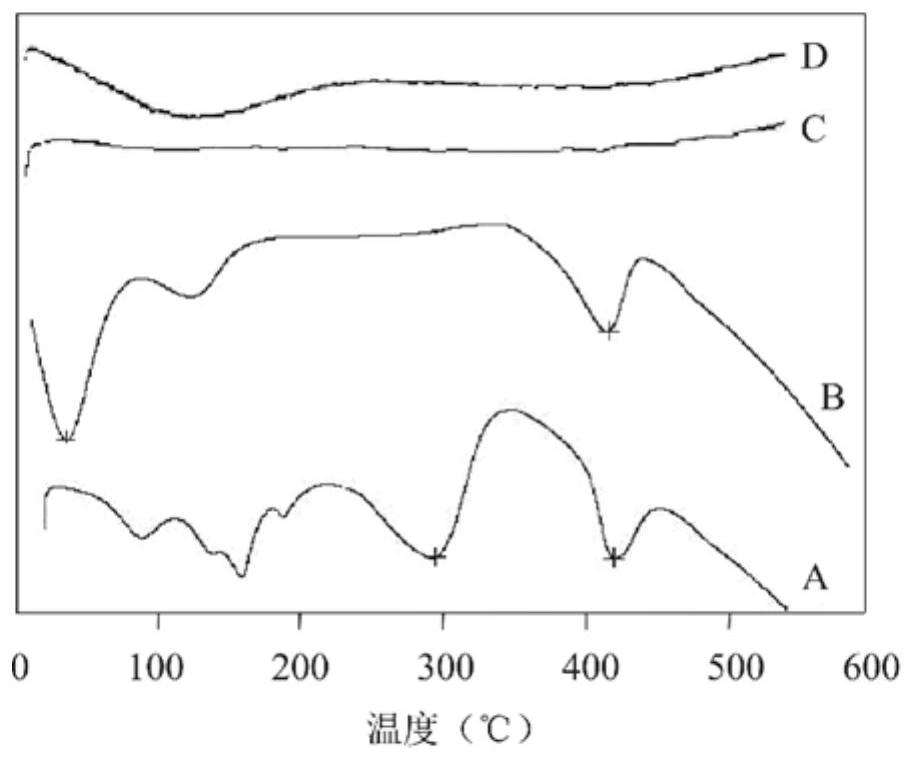
Image
Examples
Embodiment 1
[0048] 1. Selection of non-volatile solvents: non-volatile solvents need to have a certain dissolution or suspension effect on atorvastatin calcium, and choosing a solvent with a higher solubility for atorvastatin calcium is beneficial to improve the atorvastatin calcium. The dissolution rate of calcium, therefore, is screened for the non-volatile solvents by measuring their equilibrium solubility. The equilibrium solubility determination method is as follows:
[0049] Weigh the excess atorvastatin calcium raw material, place it in a 25mL conical flask, add 10mL of liquid excipients PEG400, PEG600, and propylene glycol respectively, vortex for 5min, and ultrasonicate for 30min to fully mix the drug with the liquid excipient Afterwards, place in an air bath shaker, shake at a constant temperature at 25°C, and rotate at 200 rpm / min. After 72 hours, take an appropriate amount of sample, centrifuge at 10,000rpm / min for 15min with a centrifuge, take the supernatant, dilute it with...
Embodiment 2
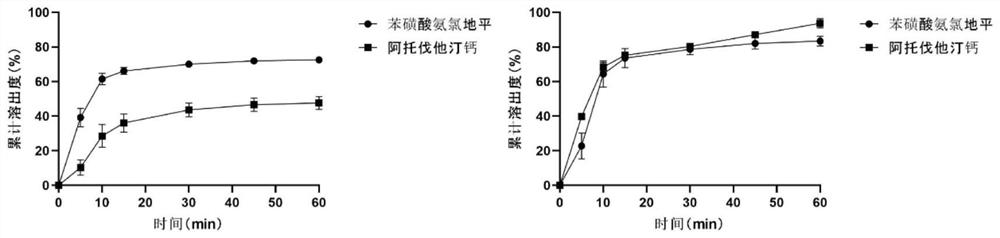
[0058] According to the result obtained in embodiment 1, determine the prescription of amlodipine atorvastatin calcium liquid-solid compressed tablet:
[0059] Table 2 Prescription 1 (amount per tablet / mg)
[0060] Atorvastatin Calcium calcium carbonate Propylene Glycol MCC-PH102 fumed silica Amlodipine Plus MCC-PH102 CMS-Na MS Sheet weight 10.8 20.0 108 616.9 30.8 6.9 127.57 50 9 980
[0061] Preparation:
[0062] (1) Add atorvastatin calcium and calcium carbonate to propylene glycol and grind evenly;
[0063] (2) Slowly drop the mixture obtained in step (1) into MCC-PH102, grind while adding, continue grinding for 1 hour until completely uniform after dropping, add fumed silica and continue grinding for 0.5 hours.
[0064] (3) Mix the material obtained in step (2) with amlodipine besylate, plus MCC-PH102, CMS-Na, and magnesium stearate, and then directly press into tablets to obtain amlodipine atorvastatin calcium solution Soli...
Embodiment 3
[0066] Table 3 Prescription 2 (amount per tablet / mg)
[0067] Atorvastatin Calcium calcium carbonate Propylene Glycol MCC-PH102 fumed silica Amlodipine Plus MCC-PH102 CMS-Na MS Sheet weight 10.8 20.0 72 456.9 22.8 6.9 162.6 40 8 800
[0068] The preparation method of this embodiment is the same as that of Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com