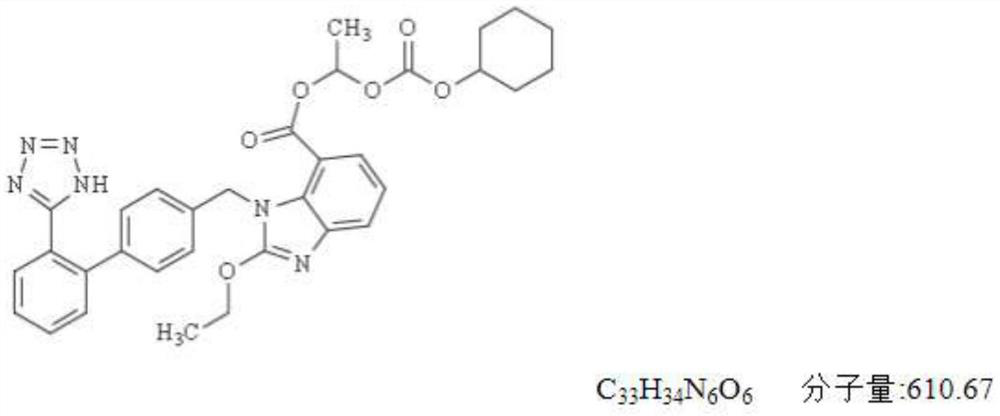
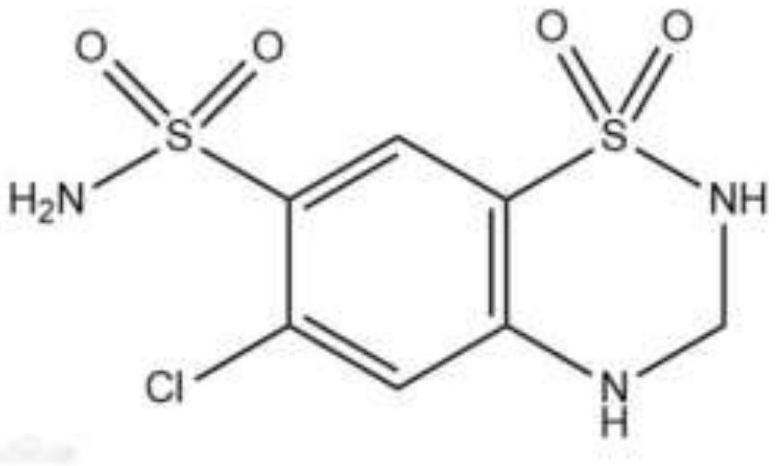
Preparation method of candesartan cilexetil and hydrochlorothiazide compound tablet
A technology of candesartan medoxomil and hydrochlorothiazide is applied in the directions of non-active ingredient medical preparations, medical preparations containing active ingredients, and pill delivery, etc. Conducive to commercial production, simple process, and the effect of improving dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The following formulations shown in Table 1 were used to compare the effects of different processes of Examples 1-5 on the in vitro dissolution and appearance of the tablet.
[0043] Table 1
[0044]
[0045]
[0046] Preparation Process:
[0047] (1) Polyethylene glycol is dissolved in 835g of water until it is completely dissolved, then hydroxypropyl cellulose is added and dissolved until clarified to obtain a binder solution added with a stabilizer;
[0048] (2) Dispersing yellow iron oxide and red iron oxide in the aqueous solution of (1) under stirring;
[0049] (3) adding candesartan cilexetil to the solution of (2) under stirring to prepare a suspension;
[0050] (4) After mixing lactose and corn starch, pass through a 30-mesh sieve and add to the fluidized bed, and spray the suspension containing candesartan cilexetil into the fluidized bed at a material temperature of 25-30°C for preparation. grain;
[0051] (5) After the granulation is finished, dry ...
Embodiment 2
[0055] Preparation Process:
[0056] (1) Dissolve polyethylene glycol in 835g of water until it is completely dissolved, then add hydroxypropyl cellulose and dissolve until clear;
[0057] (2) Dispersing yellow iron oxide and red iron oxide in the aqueous solution of (1) under stirring;
[0058] (3) After mixing candesartan cilexetil, lactose and cornstarch, pass through a 30-mesh sieve and add to the fluidized bed, and spray the prepared solution of (2) onto the fluidized bed at a material temperature of 25-30°C Carry out granulation in; (embodiment 1 is that candesartan cilexetil is added in the aqueous solution of binding agent and stabilizer, and embodiment 2 is that candesartan cilexetil and lactose, cornstarch are mixed in dry powder form and then added to in a fluidized bed);
[0059] (5) After the granulation is finished, dry until the moisture is about 1%, and pass through a 30-mesh sieve for granulation;
[0060] (6) Add hydrochlorothiazide, carmellose calcium and...
Embodiment 3
[0066] Preparation Process:
[0067] (1) Dissolve polyethylene glycol in 835g of water until it is completely dissolved, then add hydroxypropyl cellulose and dissolve until clear;
[0068] (2) Dispersing yellow iron oxide and red iron oxide in the aqueous solution of (1) under stirring;
[0069] (3) adding candesartan cilexetil to the solution of (2) under stirring to prepare a suspension;
[0070] (4) After mixing hydrochlorothiazide, lactose and corn starch, pass through a 30-mesh sieve and add to the fluidized bed, and spray the suspension containing candesartan cilexetil into the fluidized bed at a material temperature of 25-30°C to granulate;
[0071] (5) After the granulation is finished, dry until the moisture is about 1%, and pass through a 30-mesh sieve for granulation;
[0072] (6) add carmellose calcium and magnesium stearate according to particle weight and mix homogeneously;
[0073] (7) Use 8.5*5mm punched sheet to get it.
[0074] Get respectively the candi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com