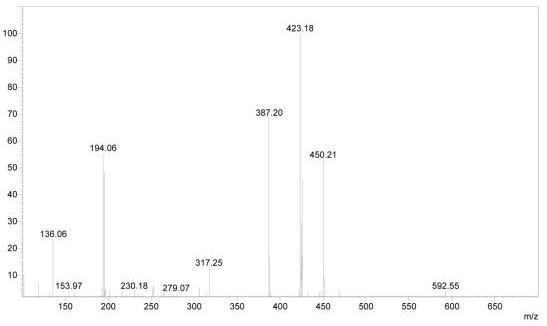
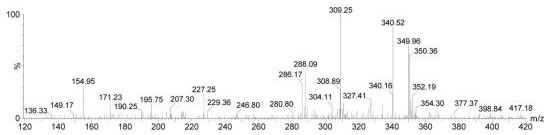
A kind of preparation method of (r)-2-(2-phosphomethoxypropyl)-adenine
A technology of methoxypropyl phosphate and adenine, which is applied in the field of preparation of -2--adenine, and can solve the problems such as preparation method, purification method and identification method that are not mentioned.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The embodiment of the present invention provides a preparation method of (R)-2-(2-phosphomethoxypropyl)-adenine, comprising:
[0033] Step S11, adding compound II, compound III and the first basic substance into the first reaction solvent, and reacting at the first temperature. After the reaction, adding ethanol to the system, separating the liquid and concentrating it to obtain a concentrated liquid; Add water and the first organic solvent to the concentrated solution, separate layers, dry the organic phase, and recrystallize to obtain intermediate IV, the compound II is adenine, the compound III is R-propylene carbonate, and the intermediate IV is (R)-(+)-2-(2-hydroxypropyl)adenine;
[0034] Step S12, adding the intermediate IV, the compound V having the structure of formula V and the catalyst into the second reaction solvent, and reacting at the second temperature. After the reaction, the temperature is lowered, the acid is adjusted to neutral pH, and ethyl acetate i...
Embodiment 1
[0053]
[0054] Step S1: Add 100.00g (0.74mol) of adenine, 90.6g (0.888mol) of R-propylene carbonate, 0.9g (0.022mol) of sodium hydroxide and 200mL of N-methylpyrrolidone into a 2L three-neck flask, and heat up to React at 125~135°C for 5 hours; after the reaction, cool the system to 80~90°C, add 1000mL of ethanol, stir slowly, cool down to room temperature, stir for 1 hour, and filter to obtain the filtrate; , to obtain a concentrated solution, add an appropriate amount of water to the concentrated solution, extract with dichloromethane to obtain an organic phase, dry the organic phase with anhydrous sodium sulfate, and finally concentrate under reduced pressure to obtain 14.5 g of a solid, add 70 mL of absolute ethanol, heat to reflux, dissolve cleared, cooled and crystallized to obtain 5.9 g of the target product (R)-(+)-2-(2-hydroxypropyl)adenine.
[0055] Step S2: Add 20.0 g (0.104 mol) of (R)-(+)-2-(2-hydroxypropyl) adenine obtained in the above step S1, magnesium ter...
Embodiment 2
[0066] Step S1: Add 100.00g (0.74mol) of adenine, 90.6g (0.888mol) of R-propylene carbonate, 0.9g (0.022mol) of sodium hydroxide and 200mL of N,N-dimethylformamide into a 2L three-neck flask , heated up to 125~135°C for reaction, and reacted for 5 hours; after the reaction, cooled the system to 80~90°C, added 1000mL of ethanol, stirred slowly, lowered to room temperature, stirred for 1 hour, and filtered to obtain the filtrate; the filtrate was concentrated to almost no liquid After dripping, a concentrated solution was obtained. Add an appropriate amount of water to the concentrated solution, extract with dichloromethane to obtain an organic phase, dry the organic phase with anhydrous sodium sulfate, and finally concentrate under reduced pressure to obtain 13.5 g of a solid, add 70 mL of absolute ethanol, and heat Reflux, dissolve, and crystallize by lowering the temperature to obtain 5.1 g of the target product (R)-(+)-2-(2-hydroxypropyl)adenine.
[0067]Step S2: Add 20.0 g ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com