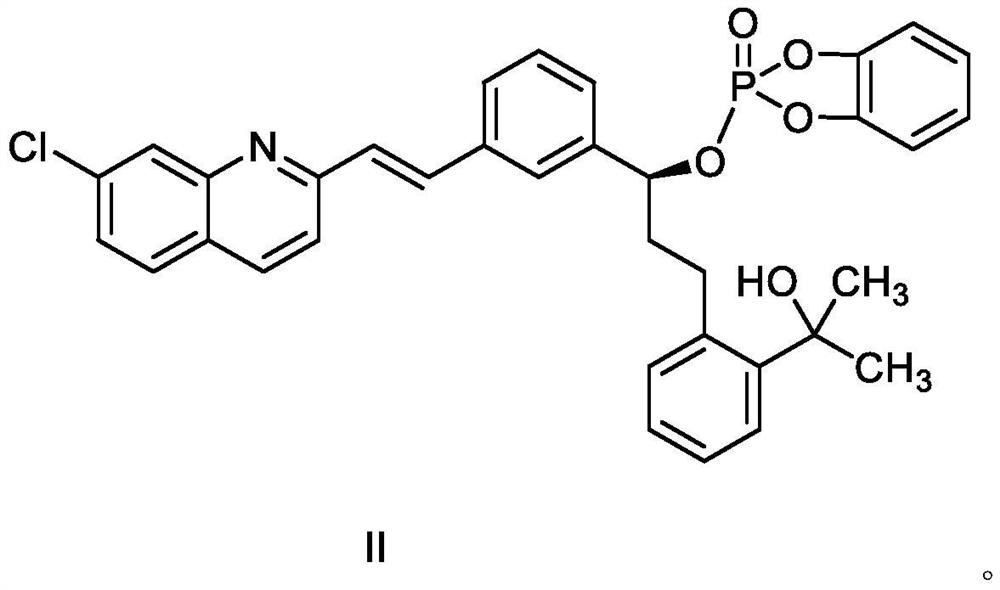
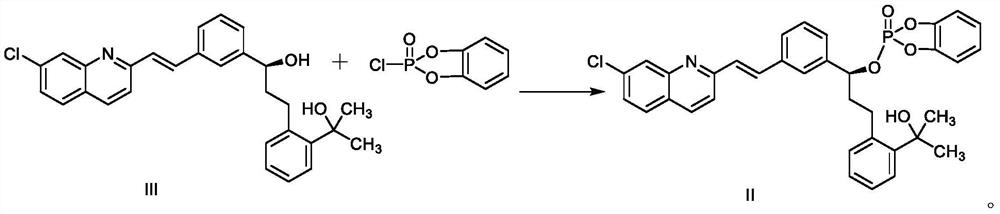
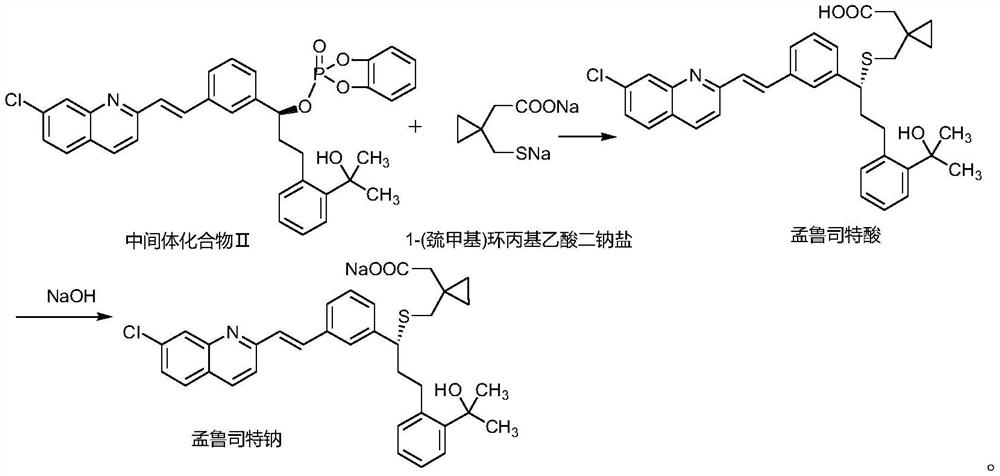
Montelukast sodium intermediate compound
A technology for montelukast sodium and its compounds, which is applied in the field of intermediate compounds of montelukast sodium, can solve the problems of troublesome post-processing, harsh operating conditions, and low purity, and achieve easy storage, good stability, and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: Preparation of Montelukast Sodium Intermediate Compound II
[0051] Under nitrogen protection, in a 1000mL there-necked flask with mechanical stirring, add 45.8g 2-(2-(3-(2-(7-chloro-2-quinolyl)-vinylphenyl)-3-hydroxypropane Base) phenyl)-2-propanol (compound III), 550mL tetrahydrofuran, 25.8g N,N-diisopropylethylamine, stirring and cooling to -10~-5°C, adding 22.9g o- The mixed solution of phenylenedioxyphosphoryl chloride and 70 mL of tetrahydrofuran was added dropwise, and stirred at -10 to -5°C for 3 to 4 hours; slowly warmed to room temperature, and the reaction progress was detected by TLC. After the reaction is completed, filter, wash the filter cake with 30mL tetrahydrofuran, evaporate the filtrate to remove the solvent under reduced pressure, add 100mL purified water and 300mL ethyl acetate to the residue, mix well and separate the liquids, and the organic layer is successively washed with 100mL 5% sodium bicarbonate aqueous solution , 100 mL of sa...
Embodiment 2
[0054] Embodiment 2: Preparation of Montelukast Sodium Intermediate Compound II
[0055] Under nitrogen protection, in a 1000mL there-necked flask with mechanical stirring, add 45.8g 2-(2-(3-(2-(7-chloro-2-quinolyl)-vinylphenyl)-3-hydroxypropane Base) phenyl)-2-propanol (compound III), 458mL acetonitrile, 18.2g triethylamine, stirring to cool down to -15~-10℃, dropwise adding 21.0g phthalic dioxyphosphoryl chloride and 70mL The tetrahydrofuran mixed solution, after the dropwise addition, was stirred and reacted at -15 to -10°C for 3 to 4 hours; it was slowly raised to room temperature, and the reaction progress was detected by TLC. After the reaction is completed, filter, wash the filter cake with 20 mL of tetrahydrofuran, evaporate the filtrate to remove the solvent under reduced pressure, add 100 mL of purified water and 300 mL of ethyl acetate to the residue, mix well and separate the liquids, and use 100 mL of 5% sodium bicarbonate aqueous solution, 100 mL of saturated br...
Embodiment 3
[0057] Embodiment 3: Preparation of Montelukast Sodium Intermediate Compound II
[0058] Under nitrogen protection, in a 1000mL three-necked flask with mechanical stirring, add 45.8g (0.1mol) 2-(2-(3-(2-(7-chloro-2-quinolyl)-vinylphenyl)- 3-hydroxypropyl)phenyl)-2-propanol (compound III), 640mL tetrahydrofuran, 26.9g DMAP, stirring and cooling down to -10~-5°C, adding 26.7g of phthalic dioxyphosphoryl chloride dropwise under stirring Mix the solution with 80mL of tetrahydrofuran, after the dropwise addition is completed, stir and react at -10 to -5°C for 3 to 4 hours; slowly rise to room temperature, and detect the reaction progress by TLC. After the reaction is completed, filter, wash the filter cake with 35mL tetrahydrofuran, evaporate the filtrate to remove the solvent under reduced pressure, add 100mL purified water and 300mL ethyl acetate to the residue, mix well and separate the liquids, and the organic layer is sequentially washed with 100mL 5% sodium bicarbonate aqueou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com